Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway
- PMID: 11598019
- PMCID: PMC125682
- DOI: 10.1093/emboj/20.20.5769
Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway
Erratum in
-
Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway.EMBO J. 2023 Jul 17;42(14):e114021. doi: 10.15252/embj.2023114021. Epub 2023 Jun 28. EMBO J. 2023. PMID: 37376956 Free PMC article.
Abstract
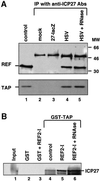
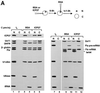
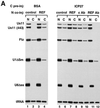
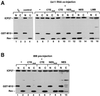
The role of herpes simplex virus ICP27 protein in mRNA export is investigated by microinjection into Xenopus laevis oocytes. ICP27 dramatically stimulates the export of intronless viral mRNAs, but has no effect on the export of cellular mRNAs, U snRNAs or tRNA. Use of inhibitors shows, in contrast to previous suggestions, that ICP27 neither shuttles nor exports viral mRNA via the CRM1 pathway. Instead, ICP27-mediated viral RNA export requires REF and TAP/NXF1, factors involved in cellular mRNA export. ICP27 binds directly to REF and complexes containing ICP27, REF and TAP are found in vitro and in virally infected cells. A mutant ICP27 that does not interact with REF is inactive in viral mRNA export. We propose that ICP27 associates with viral mRNAs and recruits TAP/NXF1 via its interaction with REF proteins, allowing the otherwise inefficiently exported viral mRNAs to access the TAP-mediated export pathway. This represents a novel mechanism for export of viral mRNAs.
Figures









Similar articles
-
The cellular RNA export receptor TAP/NXF1 is required for ICP27-mediated export of herpes simplex virus 1 RNA, but the TREX complex adaptor protein Aly/REF appears to be dispensable.J Virol. 2009 Jul;83(13):6335-46. doi: 10.1128/JVI.00375-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369354 Free PMC article.
-
ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway.J Virol. 2002 Dec;76(24):12877-89. doi: 10.1128/jvi.76.24.12877-12889.2002. J Virol. 2002. PMID: 12438613 Free PMC article.
-
ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export.J Virol. 2005 Apr;79(7):3949-61. doi: 10.1128/JVI.79.7.3949-3961.2005. J Virol. 2005. PMID: 15767397 Free PMC article.
-
The many roles of the regulatory protein ICP27 during herpes simplex virus infection.Front Biosci. 2008 May 1;13:5241-56. doi: 10.2741/3078. Front Biosci. 2008. PMID: 18508584 Review.
-
The many roles of the highly interactive HSV protein ICP27, a key regulator of infection.Future Microbiol. 2011 Nov;6(11):1261-77. doi: 10.2217/fmb.11.119. Future Microbiol. 2011. PMID: 22082288 Review.
Cited by
-
Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its subcellular distribution.J Virol. 2010 Jan;84(1):270-9. doi: 10.1128/JVI.01740-09. J Virol. 2010. PMID: 19864386 Free PMC article.
-
REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export.J Cell Biol. 2002 Nov 25;159(4):579-88. doi: 10.1083/jcb.200207128. Epub 2002 Nov 18. J Cell Biol. 2002. PMID: 12438415 Free PMC article.
-
Herpes Simplex Virus 1 Expressing GFP-Tagged Virion Host Shutoff (vhs) Protein Uncouples the Activities of RNA Degradation and Differential Nuclear Retention of the Virus Transcriptome.J Virol. 2022 Jul 27;96(14):e0192621. doi: 10.1128/jvi.01926-21. Epub 2022 Jun 27. J Virol. 2022. PMID: 35758691 Free PMC article.
-
Phenotypic suppression of a herpes simplex virus 1 ICP27 mutation by enhanced transcription of the mutant gene.J Virol. 2011 Jun;85(11):5685-90. doi: 10.1128/JVI.00315-11. Epub 2011 Mar 16. J Virol. 2011. PMID: 21411532 Free PMC article.
-
Herpes simplex virus type 1 ICP27 regulates expression of a variant, secreted form of glycoprotein C by an intron retention mechanism.J Virol. 2008 Aug;82(15):7443-55. doi: 10.1128/JVI.00388-08. Epub 2008 May 21. J Virol. 2008. PMID: 18495765 Free PMC article.
References
-
- Ace C.I., Dalrymple,M.A., Ramsay,F.H., Preston,V.G. and Preston,C.M. (1988) Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J. Gen. Virol., 69, 2595–2605. - PubMed
-
- Arts G.J., Fornerod,M. and Mattaj,I.W. (1998) Identification of a nuclear export receptor for tRNA. Curr. Biol., 8, 305–314. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

