Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis
- PMID: 11593004
- PMCID: PMC59821
- DOI: 10.1073/pnas.211190298
Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis
Abstract
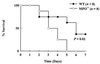
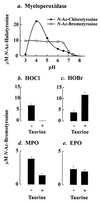
The myeloperoxidase system of neutrophils uses hydrogen peroxide and chloride to generate hypochlorous acid, a potent bactericidal oxidant in vitro. In a mouse model of polymicrobial sepsis, we observed that mice deficient in myeloperoxidase were more likely than wild-type mice to die from infection. Mass spectrometric analysis of peritoneal inflammatory fluid from septic wild-type mice detected elevated concentrations of 3-chlorotyrosine, a characteristic end product of the myeloperoxidase system. Levels of 3-chlorotyrosine did not rise in the septic myeloperoxidase-deficient mice. Thus, myeloperoxidase seems to protect against sepsis in vivo by producing halogenating species. Surprisingly, levels of 3-bromotyrosine also were elevated in peritoneal fluid from septic wild-type mice and were markedly reduced in peritoneal fluid from septic myeloperoxidase-deficient mice. Furthermore, physiologic concentrations of bromide modulated the bactericidal effects of myeloperoxidase in vitro. It seems, therefore, that myeloperoxidase can use bromide as well as chloride to produce oxidants in vivo, even though the extracellular concentration of bromide is at least 1,000-fold lower than that of chloride. Thus, myeloperoxidase plays an important role in host defense against bacterial pathogens, and bromide might be a previously unsuspected component of this system.
Figures




Similar articles
-
Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense.J Immunol. 2005 Feb 1;174(3):1557-65. doi: 10.4049/jimmunol.174.3.1557. J Immunol. 2005. PMID: 15661916
-
Myeloperoxidase produces nitrating oxidants in vivo.J Clin Invest. 2002 May;109(10):1311-9. doi: 10.1172/JCI15021. J Clin Invest. 2002. PMID: 12021246 Free PMC article.
-
Human neutrophils employ chlorine gas as an oxidant during phagocytosis.J Clin Invest. 1996 Sep 15;98(6):1283-9. doi: 10.1172/JCI118914. J Clin Invest. 1996. PMID: 8823292 Free PMC article.
-
Pathways for oxidation of low density lipoprotein by myeloperoxidase: tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine.Biofactors. 1997;6(2):145-55. doi: 10.1002/biof.5520060208. Biofactors. 1997. PMID: 9259996 Review.
-
Measuring chlorine bleach in biology and medicine.Biochim Biophys Acta. 2014 Feb;1840(2):781-93. doi: 10.1016/j.bbagen.2013.07.004. Epub 2013 Jul 18. Biochim Biophys Acta. 2014. PMID: 23872351 Review.
Cited by
-
Association between serum myeloperoxidase levels and coronary artery disease in patients without diabetes, hypertension, obesity, and hyperlipidemia.Adv Biomed Res. 2016 Jun 8;5:103. doi: 10.4103/2277-9175.183663. eCollection 2016. Adv Biomed Res. 2016. PMID: 27376042 Free PMC article.
-
Redox mechanisms in age-related lung fibrosis.Redox Biol. 2016 Oct;9:67-76. doi: 10.1016/j.redox.2016.06.005. Epub 2016 Jun 25. Redox Biol. 2016. PMID: 27394680 Free PMC article. Review.
-
Specific sequence motifs direct the oxygenation and chlorination of tryptophan by myeloperoxidase.Biochemistry. 2006 Mar 28;45(12):3961-71. doi: 10.1021/bi052339+. Biochemistry. 2006. PMID: 16548523 Free PMC article.
-
Effects of Myeloperoxidase on Methicillin-Resistant Staphylococcus aureus-Colonized Burn Wounds in Rats.Adv Wound Care (New Rochelle). 2019 Jul 1;8(7):271-280. doi: 10.1089/wound.2018.0865. Epub 2019 Jul 2. Adv Wound Care (New Rochelle). 2019. PMID: 31737417 Free PMC article.
-
Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice.J Neurosci. 2005 Jul 13;25(28):6594-600. doi: 10.1523/JNEUROSCI.0970-05.2005. J Neurosci. 2005. PMID: 16014720 Free PMC article.
References
-
- Klebanoff S. Science. 1970;169:1095–1097. - PubMed
-
- Babior B. Am J Med. 2000;109:33–44. - PubMed
-
- Harrison J E, Schultz J. J Biol Chem. 1976;251:1371–1374. - PubMed
-
- Klebanoff S. Proc Assoc Am Phys. 1999;111:383–389. - PubMed
-
- Hurst J K, Barrette W C., Jr CRC Crit Rev Biochem Mol Biol. 1989;24:271–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

