Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment
- PMID: 11581387
- PMCID: PMC114593
- DOI: 10.1128/JVI.75.21.10187-10199.2001
Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment
Abstract
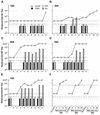
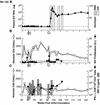
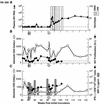
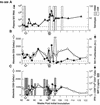
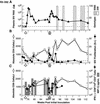
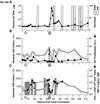
Transient antiretroviral treatment with tenofovir, (R)-9-(2-phosphonylmethoxypropyl)adenine, begun shortly after inoculation of rhesus macaques with the highly pathogenic simian immunodeficiency virus (SIV) isolate SIVsmE660, facilitated the development of SIV-specific lymphoproliferative responses and sustained effective control of the infection following drug discontinuation. Animals that controlled plasma viremia following transient postinoculation treatment showed substantial resistance to subsequent intravenous rechallenge with homologous (SIVsmE660) and highly heterologous (SIVmac239) SIV isolates, up to more than 1 year later, despite the absence of measurable neutralizing antibody. In some instances, resistance to rechallenge was observed despite the absence of detectable SIV-specific binding antibody and in the face of SIV lymphoproliferative responses that were low or undetectable at the time of challenge. In vivo monoclonal antibody depletion experiments demonstrated a critical role for CD8(+) lymphocytes in the control of viral replication; plasma viremia rose by as much as five log units after depletion of CD8(+) cells and returned to predepletion levels (as low as <100 copy Eq/ml) as circulating CD8(+) cells were restored. The extent of host control of replication of highly pathogenic SIV strains and the level of resistance to heterologous rechallenge achieved following transient postinoculation treatment compared favorably to the results seen after SIVsmE660 and SIVmac239 challenge with many vaccine strategies. This impressive control of viral replication was observed despite comparatively modest measured immune responses, less than those often achieved with vaccination regimens. The results help establish the underlying feasibility of efforts to develop vaccines for the prevention of AIDS, although the exact nature of the protective host responses involved remains to be elucidated.
Figures

Similar articles
-
Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge.J Med Primatol. 2003 Aug;32(4-5):201-10. doi: 10.1034/j.1600-0684.2003.00026.x. J Med Primatol. 2003. PMID: 14498980
-
Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment.J Virol. 2000 Mar;74(6):2584-93. doi: 10.1128/jvi.74.6.2584-2593.2000. J Virol. 2000. PMID: 10684272 Free PMC article.
-
Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques.J Virol. 2000 Feb;74(4):1704-11. doi: 10.1128/jvi.74.4.1704-1711.2000. J Virol. 2000. PMID: 10644340 Free PMC article.
-
Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques.Retrovirology. 2006 Dec 21;3:97. doi: 10.1186/1742-4690-3-97. Retrovirology. 2006. PMID: 17184540 Free PMC article.
-
Immune intervention strategies for HIV-1 infection of humans in the SIV macaque model.Vaccine. 2002 Dec 19;20 Suppl 4:A52-60. doi: 10.1016/s0264-410x(02)00388-2. Vaccine. 2002. PMID: 12477429 Review.
Cited by
-
Evolutionary dynamics of HIV at multiple spatial and temporal scales.J Mol Med (Berl). 2012 May;90(5):543-61. doi: 10.1007/s00109-012-0892-1. Epub 2012 May 3. J Mol Med (Berl). 2012. PMID: 22552382 Free PMC article. Review.
-
Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239.J Virol. 2006 Jun;80(12):5875-85. doi: 10.1128/JVI.00171-06. J Virol. 2006. PMID: 16731926 Free PMC article.
-
Similar impact of CD8+ T cell responses on early virus dynamics during SIV infections of rhesus macaques and sooty mangabeys.PLoS Comput Biol. 2010 Aug 26;6(8):e1000901. doi: 10.1371/journal.pcbi.1000901. PLoS Comput Biol. 2010. PMID: 20865048 Free PMC article.
-
Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells.Clin Vaccine Immunol. 2006 Jul;13(7):768-78. doi: 10.1128/CVI.00042-06. Clin Vaccine Immunol. 2006. PMID: 16829614 Free PMC article.
-
Emergence of simian immunodeficiency virus-specific cytotoxic CD4+ T cells and increased humoral responses correlate with control of rebounding viremia in CD8-depleted macaques infected with Rev-independent live-attenuated simian immunodeficiency virus.J Immunol. 2010 Sep 15;185(6):3348-58. doi: 10.4049/jimmunol.1000572. Epub 2010 Aug 11. J Immunol. 2010. PMID: 20702730 Free PMC article.
References
-
- Altfeld M, Rosenberg E S. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr Opin Immunol. 2000;12:375–380. - PubMed
-
- Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. - PMC - PubMed
-
- Arthur L O, Bess J W, Jr, Chertova E N, Rossio J L, Esser M T, Benveniste R E, Grimes M K, Henderson L E, Lifson J D. Simian immunodeficiency virus: production, inactivation, and characterization. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S311–S319. - PubMed
-
- Carter D L, Shieh T M, Blosser R L, Chadwick K R, Margolick J B, Hildreth J E, Clements J E, Zink M C. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. - PubMed
-
- Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV 1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:398–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

