Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells
- PMID: 11581285
- PMCID: PMC2150788
- DOI: 10.1083/jcb.200103071
Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells
Abstract
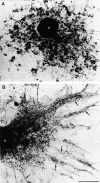
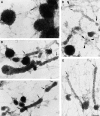
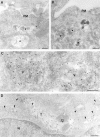
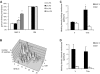
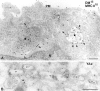
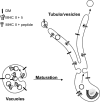
Immature dendritic cells (DCs) sample their environment for antigens and after stimulation present peptide associated with major histocompatibility complex class II (MHC II) to naive T cells. We have studied the intracellular trafficking of MHC II in cultured DCs. In immature cells, the majority of MHC II was stored intracellularly at the internal vesicles of multivesicular bodies (MVBs). In contrast, DM, an accessory molecule required for peptide loading, was located predominantly at the limiting membrane of MVBs. After stimulation, the internal vesicles carrying MHC II were transferred to the limiting membrane of the MVB, bringing MHC II and DM to the same membrane domain. Concomitantly, the MVBs transformed into long tubular organelles that extended into the periphery of the cells. Vesicles that were formed at the tips of these tubules nonselectively incorporated MHC II and DM and presumably mediated transport to the plasma membrane. We propose that in maturing DCs, the reorganization of MVBs is fundamental for the timing of MHC II antigen loading and transport to the plasma membrane.
Figures

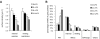
Similar articles
-
MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways.Traffic. 2009 Oct;10(10):1528-42. doi: 10.1111/j.1600-0854.2009.00963.x. Epub 2009 Jul 14. Traffic. 2009. PMID: 19682328
-
Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination.Immunity. 2006 Dec;25(6):885-94. doi: 10.1016/j.immuni.2006.11.001. Immunity. 2006. PMID: 17174123
-
Endosomally stored MHC class II does not contribute to antigen presentation by dendritic cells at inflammatory conditions.Traffic. 2011 Aug;12(8):1025-36. doi: 10.1111/j.1600-0854.2011.01212.x. Epub 2011 May 13. Traffic. 2011. PMID: 21518167
-
Endosomal sorting of MHC class II determines antigen presentation by dendritic cells.Curr Opin Cell Biol. 2008 Aug;20(4):437-44. doi: 10.1016/j.ceb.2008.05.011. Epub 2008 Jul 5. Curr Opin Cell Biol. 2008. PMID: 18582577 Review.
-
Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces.Immunol Rev. 2005 Oct;207:191-205. doi: 10.1111/j.0105-2896.2005.00317.x. Immunol Rev. 2005. PMID: 16181337 Review.
Cited by
-
Presentation of phagocytosed antigens by MHC class I and II.Traffic. 2013 Feb;14(2):135-52. doi: 10.1111/tra.12026. Epub 2012 Nov 29. Traffic. 2013. PMID: 23127154 Free PMC article. Review.
-
Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T cells.Traffic. 2011 Dec;12(12):1669-76. doi: 10.1111/j.1600-0854.2011.01251.x. Epub 2011 Aug 15. Traffic. 2011. PMID: 21762455 Free PMC article. Review.
-
The Late Endosomal Pathway Regulates the Ciliary Targeting of Tetraspanin Protein Peripherin 2.J Neurosci. 2019 May 1;39(18):3376-3393. doi: 10.1523/JNEUROSCI.2811-18.2019. Epub 2019 Feb 28. J Neurosci. 2019. PMID: 30819798 Free PMC article.
-
Cytokines regulate cysteine cathepsins during TLR responses.Cell Immunol. 2011;267(1):56-66. doi: 10.1016/j.cellimm.2010.11.004. Epub 2010 Nov 19. Cell Immunol. 2011. PMID: 21145045 Free PMC article.
-
Endolysosomal transient receptor potential mucolipins and two-pore channels: implications for cancer immunity.Front Immunol. 2024 May 22;15:1389194. doi: 10.3389/fimmu.2024.1389194. eCollection 2024. Front Immunol. 2024. PMID: 38840905 Free PMC article. Review.
References
-
- Alfonso, C., and L. Karlsson. 2000. Nonclassical MHC class II molecules. Annu. Rev. Immunol. 18:113–142. - PubMed
-
- Amigorena, S., J.R. Drake, P. Webster, and I. Mellman. 1994. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 369:113–120. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Barois, N., F. Forquet, and J. Davoust. 1997. Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cell receptor ligation and protein kinase C activation. J. Biol. Chem. 272:3641–3647. - PubMed
-
- Barois, N., F. Forquet, and J. Davoust. 1998. Actin microfilaments control the MHC class II antigen presentation pathway in B cells. J. Cell Sci. 111:1791–1800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

