CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium
- PMID: 11579131
- PMCID: PMC1947002
- DOI: 10.1046/j.1471-4159.2001.00495.x
CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium
Abstract
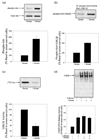
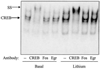
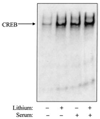
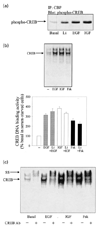
The regulatory influences of glycogen synthase kinase-3 beta (GSK3 beta) and lithium on the activity of cyclic AMP response element binding protein (CREB) were examined in human neuroblastoma SH-SY5Y cells. Activation of Akt (protein kinase B) with serum-increased phospho-serine-9-GSK3 beta (the inactive form of the enzyme), inhibited GSK3 beta activity, and increased CREB DNA binding activity. Inhibition of GSK3 beta by another paradigm, treatment with the selective inhibitor lithium, also increased CREB DNA binding activity. The inhibitory regulation of CREB DNA binding activity by GSK3 beta also was evident in differentiated SH-SY5Y cells, indicating that this regulatory interaction is maintained in non-proliferating cells. These results demonstrate that inhibition of GSK3 beta by serine-9 phosphorylation or directly by lithium increases CREB activation. Conversely, overexpression of active GSK3 beta to 3.5-fold the normal levels completely blocked increases in CREB DNA binding activity induced by epidermal growth factor, insulin-like growth factor-1, forskolin, and cyclic AMP. The inhibitory effects due to overexpressed GSK3 beta were reversed by treatment with lithium and with another GSK 3beta inhibitor, sodium valproate. Overall, these results demonstrate that GSK3 beta inhibits, and lithium enhances, CREB activation.
Figures




Similar articles
-
BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents.J Neurochem. 2002 Jul;82(1):75-83. doi: 10.1046/j.1471-4159.2002.00939.x. J Neurochem. 2002. PMID: 12091467
-
Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge.Biochemistry. 1998 Mar 17;37(11):3795-809. doi: 10.1021/bi970982t. Biochemistry. 1998. PMID: 9521699
-
Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium.Brain Res. 2001 Nov 16;919(1):106-14. doi: 10.1016/s0006-8993(01)03005-0. Brain Res. 2001. PMID: 11689167
-
Multisite phosphorylation of the cAMP response element-binding protein (CREB) by a diversity of protein kinases.Front Biosci. 2007 Jan 1;12:1814-32. doi: 10.2741/2190. Front Biosci. 2007. PMID: 17127423 Review.
-
Inhibition of glycogen synthase kinase 3 by lithium, a mechanism in search of specificity.Front Mol Neurosci. 2022 Nov 24;15:1028963. doi: 10.3389/fnmol.2022.1028963. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36504683 Free PMC article. Review.
Cited by
-
The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: An in-depth review.Front Neurosci. 2022 Sep 1;16:970925. doi: 10.3389/fnins.2022.970925. eCollection 2022. Front Neurosci. 2022. PMID: 36117625 Free PMC article. Review.
-
Lithium and GSK3-β promoter gene variants influence white matter microstructure in bipolar disorder.Neuropsychopharmacology. 2013 Jan;38(2):313-27. doi: 10.1038/npp.2012.172. Epub 2012 Sep 19. Neuropsychopharmacology. 2013. PMID: 22990942 Free PMC article.
-
BDNF reverses aging-related microglial activation.J Neuroinflammation. 2020 Jul 14;17(1):210. doi: 10.1186/s12974-020-01887-1. J Neuroinflammation. 2020. PMID: 32664974 Free PMC article.
-
RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways.PLoS One. 2010 Apr 9;5(4):e10092. doi: 10.1371/journal.pone.0010092. PLoS One. 2010. PMID: 20404919 Free PMC article.
-
Differential Roles of Accumbal GSK3β in Cocaine versus Morphine-Induced Place Preference, U50,488H-Induced Place Aversion, and Object Memory.J Pharmacol Exp Ther. 2019 Nov;371(2):339-347. doi: 10.1124/jpet.119.259283. Epub 2019 Aug 16. J Pharmacol Exp Ther. 2019. PMID: 31420527 Free PMC article.
References
-
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1933. - PubMed
-
- Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3β in the regulation of HSF-1 activity. J. Neurochem. 2000;75:2401–2408. - PubMed
-
- Bijur GN, DeSarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 2000;275:7583–7590. - PubMed
-
- Bournat JC, Brown AMC, Soler AP. Wnt-1 dependent activation of the survival factor NF-kB in PC12 cells. J. Neurosci. Res. 2000;61:21–32. - PubMed
-
- Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNAbinding activity. Cell. 1991;64:573–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

