SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma
- PMID: 11572935
- PMCID: PMC59762
- DOI: 10.1073/pnas.211026798
SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma
Abstract
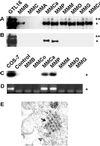
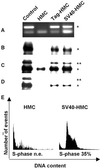
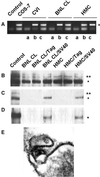
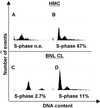
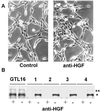
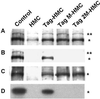
Recent studies suggested that simian virus 40 (SV40) may cause malignant mesothelioma, although the pathogenic mechanism is unclear. We found that in SV40-positive malignant mesothelioma cells, the hepatocyte growth factor (HGF) receptor (Met) was activated. In human mesothelial cells (HMC) transfected with full-length SV40 DNA (SV40-HMC), Met receptor activation was associated with S-phase entry, acquisition of a fibroblastoid morphology, and the assembly of viral particles. Coculture experiments revealed the ability of SV40-HMC to infect permissive monkey cells (CV-1), HMC, and murine BNL CL cells. Cocultured human and murine SV40-positive cells expressed HGF, showed Met tyrosine phosphorylation and S-phase entry, and acquired a spindle-shaped morphology (spBNL), whereas CV-1 cells were lysed. Cocultured HMC inherited from SV40-HMC the infectivity, as they induced lysis in cocultured CV-1 cells. Treatment with suramin or HGF-blocking antibodies inhibited Met tyrosine phosphorylation in all large T antigen (Tag)-positive cells and reverted the spindle-shaped morphology of spBNL. This finding indicated that Met activation and subsequent biological effects were mediated by an autocrine HGF circuit. This, in turn, was causally related to Tag expression, being induced by transfection with the SV40 early region alone. Our findings suggest that when SV40 infects HMC it causes Met activation via an autocrine loop. Furthermore, SV40 replicates in HMC and infects the adjacent HMC, inducing an HGF-dependent Met activation and cell-cycle progression into S phase. This may explain how a limited number of SV40-positive cells may be sufficient to direct noninfected HMC toward malignant transformation.
Figures


Similar articles
-
Different susceptibility of human mesothelial cells to polyomavirus infection and malignant transformation.Cancer Res. 2003 Oct 1;63(19):6125-9. Cancer Res. 2003. PMID: 14559789
-
Effects of asbestos on initiation of DNA damage, induction of DNA-strand breaks, P53-expression and apoptosis in primary, SV40-transformed and malignant human mesothelial cells.Mutat Res. 2004 Mar 14;558(1-2):81-92. doi: 10.1016/j.mrgentox.2003.11.003. Mutat Res. 2004. PMID: 15036122
-
SV40 infection induces telomerase activity in human mesothelial cells.Oncogene. 2002 Feb 21;21(9):1434-42. doi: 10.1038/sj.onc.1205203. Oncogene. 2002. PMID: 11857086
-
Hepatocyte growth factor and Met in tumor biology and therapeutic approach with NK4.Proteomics. 2008 Aug;8(16):3360-70. doi: 10.1002/pmic.200800156. Proteomics. 2008. PMID: 18646008 Review.
-
SV40 and cell cycle perturbations in malignant mesothelioma.Semin Cancer Biol. 2001 Feb;11(1):31-8. doi: 10.1006/scbi.2000.0344. Semin Cancer Biol. 2001. PMID: 11243897 Review.
Cited by
-
Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling.Am J Pathol. 2005 Jun;166(6):1827-40. doi: 10.1016/S0002-9440(10)62492-3. Am J Pathol. 2005. PMID: 15920167 Free PMC article.
-
Infrequent existence of simian virus 40 large T antigen DNA in malignant mesothelioma in Japan.Cancer Sci. 2006 Apr;97(4):292-5. doi: 10.1111/j.1349-7006.2006.00171.x. Cancer Sci. 2006. PMID: 16630121 Free PMC article.
-
Malignant mesothelioma: facts, myths, and hypotheses.J Cell Physiol. 2012 Jan;227(1):44-58. doi: 10.1002/jcp.22724. J Cell Physiol. 2012. PMID: 21412769 Free PMC article. Review.
-
Pathogenesis of malignant pleural mesothelioma and the role of environmental and genetic factors.J Carcinog. 2008 Jul 28;7:3. doi: 10.1186/1477-3163-7-3. J Carcinog. 2008. Retraction in: J Carcinog. 2008 Aug 08;7:4. doi: 10.1186/1477-3163-7-4. PMID: 18662397 Free PMC article. Retracted.
-
DCLK1 is correlated with MET and ERK5 expression, and associated with prognosis in malignant pleural mesothelioma.Int J Oncol. 2017 Jul;51(1):91-103. doi: 10.3892/ijo.2017.4021. Epub 2017 May 26. Int J Oncol. 2017. PMID: 28560410 Free PMC article.
References
-
- Testa J R, Pass H I, Carbone M. In: Principle and Practice of Oncology. 6th Ed. De Vita V, Hellman S, Rosemberg S, editors. Philadelphia: Lippincott; 2000. pp. 1937–1943.
-
- Robledo R, Mossman B T. J Cell Physiol. 1999;180:158–166. - PubMed
-
- Butel J, Lednicky J. J Natl Cancer Inst. 1999;91:119–134. - PubMed
-
- Levresse V, Renier A, Fleury-Feith J, Levy F, Moritz S, Vivo C, Pilatte Y, Jaurand M C. Am J Respir Cell Mol Biol. 1997;17:660–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

