Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus
- PMID: 11567076
- PMCID: PMC6762891
- DOI: 10.1523/JNEUROSCI.21-19-07848.2001
Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus
Abstract
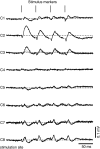
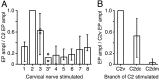
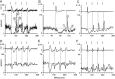
The dorsal cochlear nucleus (DCN) is a second-order auditory structure that also receives nonauditory information, including somatosensory inputs from the dorsal column and spinal trigeminal nuclei. Here we investigate the peripheral sources of the somatosensory inputs to DCN. Electrical stimulation was applied to cervical nerves C1-C8, branches of C2, branches of the trigeminal nerve, and hindlimb nerves. The largest evoked potentials in the DCN were produced by C2 stimulation and by stimulation of its branches that innervate the pinna. Electrical stimulation of C2 produced a pattern of inhibition and excitation of DCN principal cells comparable with that seen in previous studies with stimulation of the primary somatosensory nuclei, suggesting that the same pathway was activated. Because C2 contains both proprioceptive and cutaneous fibers, we applied peripheral somatosensory stimulation to identify the effective somatosensory modalities. Only stimuli that activate pinna muscle receptors, such as stretch or vibration of the muscles connected to the pinna, were effective in driving DCN units, whereas cutaneous stimuli such as light touch, brushing of hairs, and stretching of skin were ineffective. These results suggest that the largest somatosensory inputs to the DCN originate from muscle receptors associated with the pinna. They support the hypothesis that a role of the DCN in hearing is to coordinate pinna orientation to sounds or to support correction for the effects of pinna orientation on sound-localization cues.
Figures
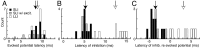
Similar articles
-
Somatosensory effects on neurons in dorsal cochlear nucleus.J Neurophysiol. 1995 Feb;73(2):743-65. doi: 10.1152/jn.1995.73.2.743. J Neurophysiol. 1995. PMID: 7760132
-
Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus.J Neurophysiol. 1996 Nov;76(5):3012-24. doi: 10.1152/jn.1996.76.5.3012. J Neurophysiol. 1996. PMID: 8930251
-
Contralateral effects and binaural interactions in dorsal cochlear nucleus.J Assoc Res Otolaryngol. 2005 Sep;6(3):280-96. doi: 10.1007/s10162-005-0008-5. J Assoc Res Otolaryngol. 2005. PMID: 16075189 Free PMC article.
-
Neural mechanisms underlying somatic tinnitus.Prog Brain Res. 2007;166:107-23. doi: 10.1016/S0079-6123(07)66010-5. Prog Brain Res. 2007. PMID: 17956776 Free PMC article. Review.
-
Somatosensory evoked potentials in man: differentiation of spinal pathways responsible for conduction from the forelimb vs hindlimb.Prog Neurobiol. 1985;25(1):1-25. doi: 10.1016/0301-0082(85)90021-8. Prog Neurobiol. 1985. PMID: 3909220 Review.
Cited by
-
Single-unit analysis of somatosensory processing in the core auditory cortex of hearing ferrets.Eur J Neurosci. 2015 Mar;41(5):686-98. doi: 10.1111/ejn.12828. Eur J Neurosci. 2015. PMID: 25728185 Free PMC article.
-
Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex.Neural Plast. 2012;2012:601591. doi: 10.1155/2012/601591. Epub 2012 Jul 19. Neural Plast. 2012. PMID: 22888454 Free PMC article.
-
Cross-modal plasticity in developmental and age-related hearing loss: Clinical implications.Hear Res. 2017 Jan;343:191-201. doi: 10.1016/j.heares.2016.08.012. Epub 2016 Sep 6. Hear Res. 2017. PMID: 27613397 Free PMC article. Review.
-
Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity.Hear Res. 2006 Jun-Jul;216-217:176-88. doi: 10.1016/j.heares.2006.03.011. Epub 2006 Apr 19. Hear Res. 2006. PMID: 16630701 Free PMC article.
-
Nonlinear temporal receptive fields of neurons in the dorsal cochlear nucleus.J Neurophysiol. 2013 Nov;110(10):2414-25. doi: 10.1152/jn.00278.2013. Epub 2013 Aug 28. J Neurophysiol. 2013. PMID: 23986561 Free PMC article.
References
-
- Abrahams VC, Richmond FJ, Keane J. Projections from C2 and C3 nerves supplying muscles and skin of the cat neck: a study using transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984b;230:142–154. - PubMed
-
- Bianconi R, Van Der Meulen JP. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963;26:177–190. - PubMed
-
- Crouch JE. Text-atlas of cat anatomy. Lea & Febiger; Philadelphia: 1969.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

