Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia
- PMID: 11567062
- PMCID: PMC6762894
- DOI: 10.1523/JNEUROSCI.21-19-07724.2001
Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia
Abstract
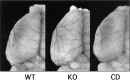
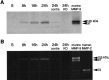
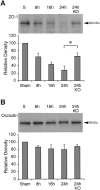
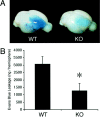
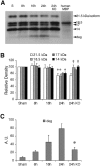
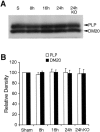
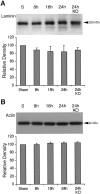
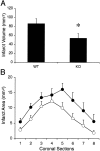
Deleterious processes of extracellular proteolysis may contribute to the progression of tissue damage after acute brain injury. We recently showed that matrix metalloproteinase-9 (MMP-9) knock-out mice were protected against ischemic and traumatic brain injury. In this study, we examined the mechanisms involved by focusing on relevant MMP-9 substrates in blood-brain barrier, matrix, and white matter. MMP-9 knock-out and wild-type mice were subjected to transient focal ischemia. MMP-9 levels increased after ischemia in wild-type brain, with expression primarily present in vascular endothelium. Western blots showed that the blood-brain barrier-associated protein and MMP-9 substrate zonae occludens-1 was degraded after ischemia, but this was reduced in knock-out mice. There were no detectable changes in another blood-brain barrier-associated protein, occludin. Correspondingly, blood-brain barrier disruption assessed via Evans Blue leakage was significantly attenuated in MMP-9 knock-out mice compared with wild types. In white matter, ischemic degradation of the MMP-9 substrate myelin basic protein was significantly reduced in knock-out mice compared with wild types, whereas there was no degradation of other myelin proteins that are not MMP substrates (proteolipid protein and DM20). There were no detectable changes in the ubiquitous structural protein actin or the extracellular matrix protein laminin. Finally, 24 hr lesion volumes were significantly reduced in knock-out mice compared with wild types. These data demonstrate that the protective effects of MMP-9 gene knock-out after transient focal ischemia may be mediated by reduced proteolytic degradation of critical blood-brain barrier and white matter components.
Figures

Similar articles
-
Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia.J Neurosci. 2007 Feb 14;27(7):1511-8. doi: 10.1523/JNEUROSCI.4391-06.2007. J Neurosci. 2007. PMID: 17301159 Free PMC article.
-
Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia.J Neurochem. 2009 Feb;108(3):811-20. doi: 10.1111/j.1471-4159.2008.05821.x. J Neurochem. 2009. PMID: 19187098 Free PMC article.
-
Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion.Brain Res. 1999 Sep 18;842(1):92-100. doi: 10.1016/s0006-8993(99)01843-0. Brain Res. 1999. PMID: 10526099
-
The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury.J Neurosurg. 2011 Jan;114(1):92-101. doi: 10.3171/2010.6.JNS10207. Epub 2010 Jul 9. J Neurosurg. 2011. PMID: 20617879
-
Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1beta levels and leakage of the blood-brain barrier.Matrix Biol. 2009 Apr;28(3):148-59. doi: 10.1016/j.matbio.2009.02.002. Epub 2009 Mar 3. Matrix Biol. 2009. PMID: 19264129 Free PMC article.
Cited by
-
The role of neutrophils in the dysfunction of central nervous system barriers.Front Aging Neurosci. 2022 Aug 11;14:965169. doi: 10.3389/fnagi.2022.965169. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36034148 Free PMC article. Review.
-
TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier.J Neuroinflammation. 2013 Jan 15;10:9. doi: 10.1186/1742-2094-10-9. J Neuroinflammation. 2013. PMID: 23320797 Free PMC article.
-
Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: mechanisms and possible interventions.Prog Neurobiol. 2012 Aug;98(2):145-65. doi: 10.1016/j.pneurobio.2012.05.010. Epub 2012 May 22. Prog Neurobiol. 2012. PMID: 22627492 Free PMC article. Review.
-
Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction.EMBO Rep. 2006 Nov;7(11):1176-81. doi: 10.1038/sj.embor.7400814. Epub 2006 Oct 6. EMBO Rep. 2006. PMID: 17028575 Free PMC article.
-
Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model.Neuroscience. 2010 Aug 11;169(1):402-14. doi: 10.1016/j.neuroscience.2010.04.043. Epub 2010 Apr 25. Neuroscience. 2010. PMID: 20423721 Free PMC article.
References
-
- Ahn MY, Zhang ZG, Tsang W, Chopp M. Endogenous plasminogen activator expression after embolic focal cerebral ischemia in mice. Brain Res. 1999;837:169–176. - PubMed
-
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator induced hemorrhage and brain injury by free radical spin trapping after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000a;20:452–457. - PubMed
-
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role of matrix metalloproteinase 9 in focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000b;20:1681–1690. - PubMed
-
- Bartus RT, Elliott PJ, Hayward NJ, Dean RL, Harbeson S, Straub JA, Li Z, Powers JC. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995;17:249–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
