Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2
- PMID: 11549730
- PMCID: PMC6762999
- DOI: 10.1523/JNEUROSCI.21-18-07194.2001
Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2
Abstract
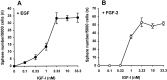
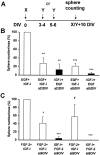
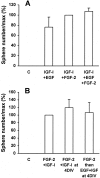
Neural stem cells (NSCs), when stimulated with epidermal growth factor (EGF) or fibroblast growth factor-2 (FGF-2), have the capacity to renew, expand, and produce precursors for neurons, astrocytes, and oligodendrocytes. We postulated that the early appearance of insulin-like growth factor (IGF-I) receptors during mouse striatum development implies a role in NSC regulation. Thus, we tested in vitro the action of IGF-I on the proliferation of striatal NSCs. In the absence of IGF-I, neither EGF nor FGF-2 was able to induce the proliferation of E14 mouse striatal cells. However, addition of IGF-I generated large proliferative clusters, termed spheres, in a dose-dependent manner. The newly generated spheres were multipotent, and clonal analysis revealed that EGF or FGF-2, in the presence of IGF-I, acted directly on NSCs. The actions of IGF-I suggest distinct modes of action of EGF or FGF-2 on NSCs. First, continuous versus delayed administration of these neurotrophic factors showed that neither IGF-I nor EGF had an effect on NSC survival, whereas FGF-2 promoted the survival or maintenance of the stem cell state of 50% of NSCs for 6 d. Second, short-term exposure to IGF-I induced the proliferation of NSCs in the presence of EGF, but not of FGF-2, through an autocrine secretion of IGF-I. These findings suggest that IGF-I is a key factor in the regulation of NSC activation and that EGF and FGF-2 control striatal NSC proliferation, in part, through distinct intracellular mechanisms.
Figures

Similar articles
-
Developmental expression of fibroblast growth factor (FGF) receptors in neural stem cell progeny. Modulation of neuronal and glial lineages by basic FGF treatment.Neurol Res. 2001 Sep;23(6):612-21. doi: 10.1179/016164101101199090. Neurol Res. 2001. PMID: 11547930
-
IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells.Int J Med Sci. 2013;10(5):522-31. doi: 10.7150/ijms.5325. Epub 2013 Mar 13. Int J Med Sci. 2013. PMID: 23532711 Free PMC article.
-
Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia.J Neurosci. 2003 Feb 1;23(3):895-906. doi: 10.1523/JNEUROSCI.23-03-00895.2003. J Neurosci. 2003. PMID: 12574418 Free PMC article.
-
Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor.J Neurosci. 1998 Mar 15;18(6):2118-28. doi: 10.1523/JNEUROSCI.18-06-02118.1998. J Neurosci. 1998. PMID: 9482798 Free PMC article. Review.
-
Epidermal Growth Factor in the CNS: A Beguiling Journey from Integrated Cell Biology to Multiple Sclerosis. An Extensive Translational Overview.Cell Mol Neurobiol. 2022 May;42(4):891-916. doi: 10.1007/s10571-020-00989-x. Epub 2020 Nov 5. Cell Mol Neurobiol. 2022. PMID: 33151415 Free PMC article. Review.
Cited by
-
Neurodevelopmental effects of insulin-like growth factor signaling.Front Neuroendocrinol. 2012 Aug;33(3):230-51. doi: 10.1016/j.yfrne.2012.06.002. Epub 2012 Jun 16. Front Neuroendocrinol. 2012. PMID: 22710100 Free PMC article. Review.
-
Expression change of stem cell-derived neural stem/progenitor cell supporting factor gene in injured spinal cord of rats.Neurosci Bull. 2007 May;23(3):165-9. doi: 10.1007/s12264-007-0024-z. Neurosci Bull. 2007. PMID: 17612595 Free PMC article.
-
MicroRNA profiling reveals unique miRNA signatures in IGF-1 treated embryonic striatal stem cell fate decisions in striatal neurogenesis in vitro.Biomed Res Int. 2014;2014:503162. doi: 10.1155/2014/503162. Epub 2014 Sep 1. Biomed Res Int. 2014. PMID: 25254208 Free PMC article.
-
Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules.Regen Biomater. 2015 Mar;2(1):31-45. doi: 10.1093/rb/rbu017. Epub 2015 Jan 13. Regen Biomater. 2015. PMID: 26813399 Free PMC article. Review.
-
CXCR7 Participates in CXCL12-mediated Cell Cycle and Proliferation Regulation in Mouse Neural Progenitor Cells.Curr Mol Med. 2016;16(8):738-746. doi: 10.2174/1566524016666160829153453. Curr Mol Med. 2016. PMID: 27573194 Free PMC article.
References
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
-
- Bartlett WP, Li X-S, Williams M, Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol. 1991;147:239–250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
