Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene
- PMID: 11544177
- PMCID: PMC312770
- DOI: 10.1101/gad.913901
Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene
Abstract
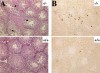
The serine/threonine kinase Akt has been implicated in the control of cell survival and metabolism. Here we report the disruption of the most ubiquitously expressed member of the akt family of genes, akt1, in the mouse. Akt1(-/-) mice are viable but smaller when compared to wild-type littermates. In addition, the life span of Akt1(-/-) mice, upon exposure to genotoxic stress, is shorter. However, Akt1(-/-) mice do not display a diabetic phenotype. Increased spontaneous apoptosis in testes, and attenuation of spermatogenesis is observed in Akt1(-/-) male mice. Increased spontaneous apoptosis is also observed in the thymi of Akt1(-/-) mice, and Akt1(-/-) thymocytes are more sensitive to apoptosis induced by gamma-irradiation and dexamethasone. Finally, Akt1(-/-) mouse embryo fibroblasts (MEFs) are more susceptible to apoptosis induced by TNF, anti-Fas, UV irradiation, and serum withdrawal.
Figures







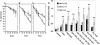
Similar articles
-
Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis.Anticancer Res. 2000 Jan-Feb;20(1A):407-16. Anticancer Res. 2000. PMID: 10769688
-
Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis.Plant Physiol. 2001 Nov;127(3):1012-9. Plant Physiol. 2001. PMID: 11706182 Free PMC article.
-
Akt1 suppresses radiation-induced germ cell apoptosis in vivo.Endocrinology. 2006 Sep;147(9):4213-21. doi: 10.1210/en.2006-0174. Epub 2006 Jun 8. Endocrinology. 2006. PMID: 16763066
-
Tumor necrosis factor-alpha and CD95 ligation suppress erythropoiesis in Fanconi anemia C gene knockout mice.J Cell Physiol. 1999 Apr;179(1):79-86. doi: 10.1002/(SICI)1097-4652(199904)179:1<79::AID-JCP10>3.0.CO;2-O. J Cell Physiol. 1999. PMID: 10082135
-
Akt2, but not Akt1, is required for cell survival by inhibiting activation of JNK and p38 after UV irradiation.Oncogene. 2009 Mar 5;28(9):1241-7. doi: 10.1038/onc.2008.487. Epub 2009 Jan 19. Oncogene. 2009. PMID: 19151757
Cited by
-
Akt: a double-edged sword in cell proliferation and genome stability.J Oncol. 2012;2012:951724. doi: 10.1155/2012/951724. Epub 2012 Mar 15. J Oncol. 2012. PMID: 22481935 Free PMC article.
-
Regulation of Hematopoietic Stem Cell Fate and Malignancy.Int J Mol Sci. 2020 Jul 6;21(13):4780. doi: 10.3390/ijms21134780. Int J Mol Sci. 2020. PMID: 32640596 Free PMC article. Review.
-
Regulation of DNA Damage Response and Homologous Recombination Repair by microRNA in Human Cells Exposed to Ionizing Radiation.Cancers (Basel). 2020 Jul 8;12(7):1838. doi: 10.3390/cancers12071838. Cancers (Basel). 2020. PMID: 32650508 Free PMC article. Review.
-
AKT1 Activation is Obligatory for Spontaneous BCC Tumor Growth in a Murine Model that Mimics Some Features of Basal Cell Nevus Syndrome.Cancer Prev Res (Phila). 2016 Oct;9(10):794-802. doi: 10.1158/1940-6207.CAPR-16-0066. Epub 2016 Jul 7. Cancer Prev Res (Phila). 2016. PMID: 27388747 Free PMC article.
-
Impaired Mitochondrial Fat Oxidation Induces FGF21 in Muscle.Cell Rep. 2016 May 24;15(8):1686-99. doi: 10.1016/j.celrep.2016.04.057. Epub 2016 May 12. Cell Rep. 2016. PMID: 27184848 Free PMC article.
References
-
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. - PubMed
-
- Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;116:2407–2411. - PubMed
-
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock J, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. - PubMed
-
- Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat Genet. 2000;24:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
