A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis
- PMID: 11535634
- PMCID: PMC2195947
- DOI: 10.1084/jem.194.5.669
A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis
Abstract
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS) characterized by plaques of infiltrating CD4(+) and CD8(+) T cells. Studies of MS and experimental autoimmune encephalomyelitis (EAE), an animal model of MS, focus on the contribution of CD4(+) myelin-specific T cells. The role of CD8(+) myelin-specific T cells in mediating EAE or MS has not been described previously. Here, we demonstrate that myelin-specific CD8(+) T cells induce severe CNS autoimmunity in mice. The pathology and clinical symptoms in CD8(+) T cell-mediated CNS autoimmunity demonstrate similarities to MS not seen in myelin-specific CD4(+) T cell-mediated EAE. These data suggest that myelin-specific CD8(+) T cells could function as effector cells in the pathogenesis of MS.
Figures
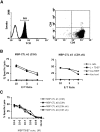
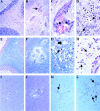
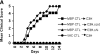
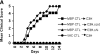
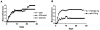
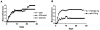
Comment in
-
Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis.J Exp Med. 2001 Sep 3;194(5):F27-30. doi: 10.1084/jem.194.5.f27. J Exp Med. 2001. PMID: 11535639 Free PMC article. Review. No abstract available.
Similar articles
-
Inconsistence between number and function of autoreactive T cells in the course of experimental autoimmune encephalomyelitis.Immunol Invest. 2018 Jan;47(1):1-17. doi: 10.1080/08820139.2017.1367008. Epub 2017 Sep 5. Immunol Invest. 2018. PMID: 28872930
-
MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8⁺ T cells.Nat Immunol. 2013 Mar;14(3):254-61. doi: 10.1038/ni.2513. Epub 2013 Jan 6. Nat Immunol. 2013. PMID: 23291597 Free PMC article.
-
The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells.J Immunol. 2013 May 15;190(10):4991-9. doi: 10.4049/jimmunol.1300083. Epub 2013 Apr 5. J Immunol. 2013. PMID: 23562810 Free PMC article.
-
Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies.Crit Rev Immunol. 1997;17(1):33-75. doi: 10.1615/critrevimmunol.v17.i1.20. Crit Rev Immunol. 1997. PMID: 9034723 Review.
-
Mechanisms regulating regional localization of inflammation during CNS autoimmunity.Immunol Rev. 2012 Jul;248(1):205-15. doi: 10.1111/j.1600-065X.2012.01126.x. Immunol Rev. 2012. PMID: 22725963 Free PMC article. Review.
Cited by
-
Microglia play a major role in direct viral-induced demyelination.Clin Dev Immunol. 2013;2013:510396. doi: 10.1155/2013/510396. Epub 2013 Jun 20. Clin Dev Immunol. 2013. PMID: 23864878 Free PMC article.
-
HSP70 and HSP90 Differentially Regulate Translocation of Extracellular Antigen to the Cytosol for Cross-Presentation.Autoimmune Dis. 2012;2012:745962. doi: 10.1155/2012/745962. Epub 2012 Sep 25. Autoimmune Dis. 2012. PMID: 23050124 Free PMC article.
-
A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice.J Virol. 2007 Dec;81(23):12962-72. doi: 10.1128/JVI.01442-07. Epub 2007 Sep 26. J Virol. 2007. PMID: 17898051 Free PMC article.
-
Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor.Br J Pharmacol. 2008 Jan;153(2):271-6. doi: 10.1038/sj.bjp.0707493. Epub 2007 Oct 8. Br J Pharmacol. 2008. PMID: 17922025 Free PMC article. Review.
-
Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus.J Virol. 2005 Aug;79(15):9388-96. doi: 10.1128/JVI.79.15.9388-9396.2005. J Virol. 2005. PMID: 16014902 Free PMC article.
References
-
- Steinman L. Multiple sclerosisa coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. - PubMed
-
- Whitaker J.N., Mitchell G.W. Clinical features of multiple sclerosis Raine C.S., McFarland H., Tourtellotte W.W. Multiple SclerosisClinical and Pathogenetic Basis 1997. 3 19 Chapman and Hall; London: pp.
-
- Traugott U., Reinherz E.L., Raine C.S. Multiple sclerosisdistribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. - PubMed
-
- Hauser S.L., Bhan A.K., Gilles F., Kemp M., Kerr C., Weiner H.L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 1986;19:578–587. - PubMed
-
- Esiri M.M., Gay D. The immunocytochemistry of multiple sclerosis plaques Raine C.S., McFarland H.F., Tourtellotte W.W. Multiple SclerosisClinical and Pathogenetic Basis 1997. 173 186 Chapman and Hall Medical; London: pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

