The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation
- PMID: 11532957
- PMCID: PMC125268
- DOI: 10.1093/emboj/20.17.4935
The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation
Abstract
Wnt-induced formation of nuclear Tcf-beta-catenin complexes promotes transcriptional activation of target genes involved in cell fate decisions. Inappropriate expression of Tcf target genes resulting from mutational activation of this pathway is also implicated in tumorigenesis. The C-terminus of beta-catenin is indispensable for the transactivation function, which probably reflects the presence of binding sites for essential transcriptional coactivators such as p300/CBP. However, the precise mechanism of transactivation remains unclear. Here we demonstrate an interaction between beta-catenin and Brg-1, a component of mammalian SWI/SNF and Rsc chromatin-remodelling complexes. A functional consequence of reintroduction of Brg-1 into Brg-1-deficient cells is enhanced activity of a Tcf-responsive reporter gene. Consistent with this, stable expression of inactive forms of Brg-1 in colon carcinoma cell lines specifically inhibits expression of endogenous Tcf target genes. In addition, we observe genetic interactions between the Brg-1 and beta-catenin homologues in flies. We conclude that beta-catenin recruits Brg-1 to Tcf target gene promoters, facilitating chromatin remodelling as a prerequisite for transcriptional activation.
Figures


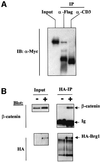
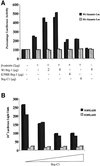


Similar articles
-
The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression.J Cell Biol. 2000 Apr 17;149(2):249-54. doi: 10.1083/jcb.149.2.249. J Cell Biol. 2000. PMID: 10769018 Free PMC article.
-
Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin.Mol Cell Biol. 2004 Dec;24(24):10689-702. doi: 10.1128/MCB.24.24.10689-10702.2004. Mol Cell Biol. 2004. PMID: 15572674 Free PMC article.
-
The Yin-Yang of TCF/beta-catenin signaling.Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
-
Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma.Science. 1997 Mar 21;275(5307):1784-7. doi: 10.1126/science.275.5307.1784. Science. 1997. PMID: 9065401
-
TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling.Biol Chem. 2002 Feb;383(2):255-61. doi: 10.1515/BC.2002.027. Biol Chem. 2002. PMID: 11934263 Review.
Cited by
-
Tumor necrosis factor α-induced hypoxia-inducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling.Mol Cell Biol. 2013 Jul;33(14):2718-31. doi: 10.1128/MCB.01254-12. Epub 2013 May 13. Mol Cell Biol. 2013. PMID: 23671189 Free PMC article.
-
IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype.J Biol Chem. 2010 Jan 15;285(3):1928-38. doi: 10.1074/jbc.M109.060319. Epub 2009 Oct 20. J Biol Chem. 2010. PMID: 19843521 Free PMC article.
-
The BAF complex inhibitor pyrimethamine reverses HIV-1 latency in people with HIV-1 on antiretroviral therapy.Sci Adv. 2023 Mar 17;9(11):eade6675. doi: 10.1126/sciadv.ade6675. Epub 2023 Mar 15. Sci Adv. 2023. PMID: 36921041 Free PMC article. Clinical Trial.
-
The ISWI-containing NURF complex regulates the output of the canonical Wingless pathway.EMBO Rep. 2009 Oct;10(10):1140-6. doi: 10.1038/embor.2009.157. Epub 2009 Aug 28. EMBO Rep. 2009. PMID: 19713963 Free PMC article.
-
Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt.Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15370-5. doi: 10.1073/pnas.0406499101. Epub 2004 Oct 18. Proc Natl Acad Sci U S A. 2004. PMID: 15492215 Free PMC article.
References
-
- Adams C.C. and Workman,J.L. (1993) Nucleosome displacement in transcription. Cell, 72, 305–308. - PubMed
-
- Behrens J., von Kries,J.P., Kuhl,M., Bruhn,L., Wedlich,D., Grosschedl,R. and Birchmeier,W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature, 382, 638–642. - PubMed
-
- Bienz M. and Clevers,H. (2000) Linking colorectal cancer to Wnt signaling. Cell, 103, 311–320. - PubMed
-
- Blomquist P., Li,Q. and Wrange,O. (1996) The affinity of nuclear factor 1 for its DNA site is drastically reduced by nucleosome organization irrespective of its rotational or translational position. J. Biol. Chem., 271, 153–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

