Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana
- PMID: 11532937
- PMCID: PMC125591
- DOI: 10.1093/emboj/20.17.4730
Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana
Abstract
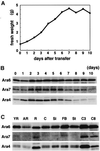
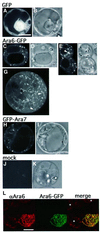
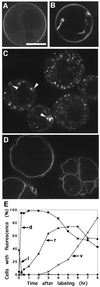
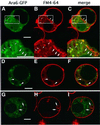
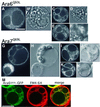
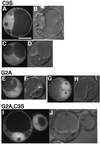
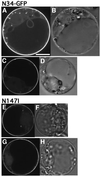
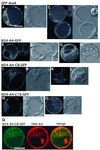
Ara6 of Arabidopsis thaliana is a novel member of the Rab/Ypt GTPase family with unique structural features. It resembles Rab5 GTPases best, but lacks a large part of the C-terminal hypervariable region and the cysteine motif, and instead harbors an extra stretch of amino acid residues containing myristoylation and palmitoylation sites at the N-terminus. Ara6 is tightly associated with membranes and is expressed constitutively. In contrast, the conventional Rab5 ortholog, Ara7, is highly expressed only in actively dividing cells. Examination of green fluorescent protein (GFP)-tagged proteins indicates that both Ara6 and Ara7 are distributed on a subpopulation of endosomes and suggests their roles in endosomal fusion. The endosomal localization of Ara6 requires N-terminal fatty acylation, nucleotide binding and the C-terminal amino acid sequence coordinately. Proteins similar to Ara6 are found only in higher plants and thus represent a novel class of Rab GTPases regulating endocytic function in a plant- specific manner.
Figures

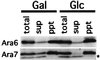

Similar articles
-
Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes.Curr Biol. 2003 Aug 19;13(16):1378-87. doi: 10.1016/s0960-9822(03)00538-4. Curr Biol. 2003. PMID: 12932321
-
Molecular and biochemical analysis of the first ARA6 homologue, a RAB5 GTPase, from green algae.J Exp Bot. 2013 Dec;64(18):5553-68. doi: 10.1093/jxb/ert322. Epub 2013 Oct 14. J Exp Bot. 2013. PMID: 24127512 Free PMC article.
-
Functional analyses of the plant-specific C-terminal region of VPS9a: the activating factor for RAB5 in Arabidopsis thaliana.J Plant Res. 2016 Jan;129(1):93-102. doi: 10.1007/s10265-015-0760-5. J Plant Res. 2016. PMID: 26493488
-
The full complement of yeast Ypt/Rab-GTPases and their involvement in exo- and endocytic trafficking.Subcell Biochem. 2000;34:133-73. doi: 10.1007/0-306-46824-7_4. Subcell Biochem. 2000. PMID: 10808333 Review. No abstract available.
-
RAB GTPases and their effectors in plant endosomal transport.Curr Opin Plant Biol. 2019 Dec;52:61-68. doi: 10.1016/j.pbi.2019.07.007. Epub 2019 Aug 24. Curr Opin Plant Biol. 2019. PMID: 31454706 Review.
Cited by
-
Cell polarity and patterning by PIN trafficking through early endosomal compartments in Arabidopsis thaliana.PLoS Genet. 2013 May;9(5):e1003540. doi: 10.1371/journal.pgen.1003540. Epub 2013 May 30. PLoS Genet. 2013. PMID: 23737757 Free PMC article.
-
The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis.Plant Cell. 2005 Sep;17(9):2554-63. doi: 10.1105/tpc.105.031237. Epub 2005 Aug 12. Plant Cell. 2005. PMID: 16100337 Free PMC article.
-
PpRab1, a Rab GTPase from maritime pine is differentially expressed during embryogenesis.Mol Genet Genomics. 2007 Sep;278(3):273-82. doi: 10.1007/s00438-007-0247-8. Epub 2007 Jun 12. Mol Genet Genomics. 2007. PMID: 17562081
-
RAB GTPases and SNAREs at the trans-Golgi network in plants.J Plant Res. 2022 May;135(3):389-403. doi: 10.1007/s10265-022-01392-x. Epub 2022 Apr 29. J Plant Res. 2022. PMID: 35488138 Free PMC article. Review.
-
Cellular and developmental function of ACAP type ARF-GAP proteins are diverged in plant cells.Plant Biotechnol (Tokyo). 2016;33(4):309-314. doi: 10.5511/plantbiotechnology.16.0309a. Epub 2016 Apr 21. Plant Biotechnol (Tokyo). 2016. PMID: 31274992 Free PMC article.
References
-
- Anai T., Hasegawa,K., Watanabe,Y., Uchimiya,H., Ishizaki,R. and Matsui,M. (1991) Isolation and analysis of cDNAs encoding small GTP-binding proteins of Arabidopsis thaliana. Gene, 108, 259–264. - PubMed
-
- Anai T., Aspuria,E.T., Fujii,N., Ueda,T., Matsui,M., Hasegawa,K. and Uchimiya,H. (1995) Immunological analysis of a small GTP-binding protein in higher plant cells. J. Plant Physiol., 147, 48–52.
-
- Anuntalabhochai S., Terryn,N., Van Montagu,M. and Inze,D. (1991) Molecular characterization of an Arabidopsis thaliana cDNA encoding a small GTP-binding protein, Rha1. Plant J., 1, 167–174. - PubMed
-
- Bolte S., Schiene,K. and Dietz,K.J. (2000) Characterization of a small GTP-binding protein of the rab5 family in Mesembryanthemum crystallinum with increased level of expression during early salt stress. Plant Mol. Biol., 42, 923–936. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

