Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels
- PMID: 11518729
- PMCID: PMC209399
- DOI: 10.1172/JCI12440
Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels
Abstract
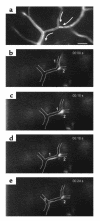
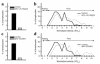
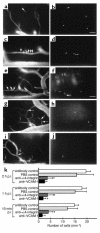
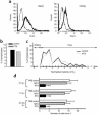
Direct in vivo evidence is still lacking for alpha4-integrin-mediated T cell interaction with VCAM-1 on blood-brain barrier-endothelium in experimental autoimmune encephalomyelitis (EAE). To investigate a possible alpha4-integrin-mediated interaction of encephalitogenic T cell blasts with VCAM-1 on the blood-brain barrier white matter endothelium in vivo, we have developed a novel spinal cord window preparation that enabled us to directly visualize CNS white matter microcirculation by intravital fluorescence videomicroscopy. Our study provides the first in vivo evidence that encephalitogenic T cell blasts interact with the spinal cord white matter microvasculature without rolling and that alpha4-integrin mediates the G protein-independent capture and subsequently the G protein-dependent adhesion strengthening of T cell blasts to microvascular VCAM-1.
Figures

Comment in
-
Encephalitogenic lymphoblast recruitment to resting CNS microvasculature: a natural immunosurveillance mechanism?J Clin Invest. 2001 Aug;108(4):517-9. doi: 10.1172/JCI13646. J Clin Invest. 2001. PMID: 11518723 Free PMC article. No abstract available.
Similar articles
-
Encephalitogenic T cells use LFA-1 for transendothelial migration but not during capture and initial adhesion strengthening in healthy spinal cord microvessels in vivo.Eur J Immunol. 2002 Dec;32(12):3598-606. doi: 10.1002/1521-4141(200212)32:12<3598::AID-IMMU3598>3.0.CO;2-6. Eur J Immunol. 2002. PMID: 12516546
-
Molecular mechanisms involved in T cell migration across the blood-brain barrier.J Neural Transm (Vienna). 2006 Apr;113(4):477-85. doi: 10.1007/s00702-005-0409-y. J Neural Transm (Vienna). 2006. PMID: 16550326 Review.
-
The development of experimental autoimmune encephalomyelitis in the mouse requires alpha4-integrin but not alpha4beta7-integrin.J Clin Invest. 1998 Dec 15;102(12):2096-105. doi: 10.1172/JCI4271. J Clin Invest. 1998. PMID: 9854045 Free PMC article.
-
Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis.Eur J Immunol. 2002 Aug;32(8):2133-44. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. Eur J Immunol. 2002. PMID: 12209625
-
Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines.J Neurol Sci. 2008 Nov 15;274(1-2):23-6. doi: 10.1016/j.jns.2008.05.019. Epub 2008 Jun 24. J Neurol Sci. 2008. PMID: 18573502 Review.
Cited by
-
The anatomical and cellular basis of immune surveillance in the central nervous system.Nat Rev Immunol. 2012 Sep;12(9):623-35. doi: 10.1038/nri3265. Epub 2012 Aug 20. Nat Rev Immunol. 2012. PMID: 22903150 Review.
-
Molecular and cellular mechanisms of pulmonary fibrosis.Fibrogenesis Tissue Repair. 2012 Jul 23;5(1):11. doi: 10.1186/1755-1536-5-11. Fibrogenesis Tissue Repair. 2012. PMID: 22824096 Free PMC article.
-
In vivo coherent anti-Stokes Raman scattering imaging of sciatic nerve tissue.J Microsc. 2007 Feb;225(Pt 2):175-82. doi: 10.1111/j.1365-2818.2007.01729.x. J Microsc. 2007. PMID: 17359252 Free PMC article.
-
Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways.J Exp Med. 2003 May 19;197(10):1255-67. doi: 10.1084/jem.20010685. J Exp Med. 2003. PMID: 12756264 Free PMC article.
-
The role of alpha-4 integrin in the aetiology of multiple sclerosis: current knowledge and therapeutic implications.CNS Drugs. 2005;19(11):909-22. doi: 10.2165/00023210-200519110-00002. CNS Drugs. 2005. PMID: 16268663 Review.
References
-
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. - PubMed
-
- Barkalow FJ, Goodman MJ, Gerritsen ME, Mayadas TN. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996;88:4585–4593. - PubMed
-
- Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. - PubMed
-
- Laschinger M, Engelhardt B. Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102:32–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

