Alcohol hypersensitivity, increased locomotion, and spontaneous myoclonus in mice lacking the potassium channels Kv3.1 and Kv3.3
- PMID: 11517255
- PMCID: PMC6763102
- DOI: 10.1523/JNEUROSCI.21-17-06657.2001
Alcohol hypersensitivity, increased locomotion, and spontaneous myoclonus in mice lacking the potassium channels Kv3.1 and Kv3.3
Abstract
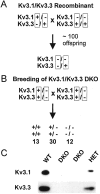
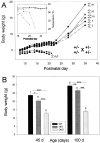
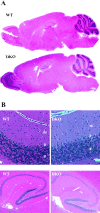
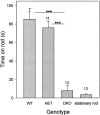
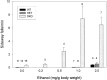
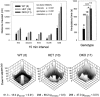
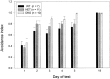
The Shaw-like potassium (K(+)) channels Kv3.1 and Kv3.3 are widely coexpressed in distinct neuronal populations in the CNS, possibly explaining the relatively "mild" phenotypes of the Kv3.1 and the Kv3.3 single mutant. Kv3.1-deficient mice show increased cortical gamma- and decreased delta-oscillations (Joho et al., 1997, 1999); otherwise, the Kv3.1-mutant phenotype is relatively subtle (Ho et al., 1997; Sánchez et al., 2000). Kv3.3-deficient mice display no overt phenotype (Chan, 1997). To investigate whether Kv3.1 and Kv3.3 K(+) channels are functionally redundant, we generated the Kv3.1/Kv3.3 double mutant. Kv3.1/Kv3.3-deficient mice were born at the expected Mendelian frequencies indicating that neither Kv3.1 nor Kv3.3 K(+) channels are essential for embryonic development. Although there are no obvious changes in gross brain anatomy, adult Kv3.1/Kv3.3-deficient mice display severe ataxia, tremulous movements, myoclonus, and hypersensitivity to ethanol. Mice appear unbalanced when moving, whereas at rest they exhibit whole-body jerks every few seconds. In spite of the severe motor impairment, Kv3.1/Kv3.3-deficient mice are hyperactive, show increased exploratory activity, and display no obvious learning or memory deficit. Myoclonus, tremor, and ethanol hypersensitivity are only seen in the double-homozygous Kv3.1/Kv3.3-deficient mice, whereas increased locomotor and exploratory activity are also present in double-heterozygous mice. The graded penetrance of mutant traits appears to depend on the number of null alleles, suggesting that some of the distinct phenotypic traits visible in the absence of Kv3.1 and Kv3.3 K(+) channels are unrelated and may be caused by localized dysfunction in different brain regions.
Figures
Similar articles
-
Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels.Genes Brain Behav. 2004 Apr;3(2):90-100. doi: 10.1046/j.1601-183x.2003.00054.x. Genes Brain Behav. 2004. PMID: 15005717
-
Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3.Eur J Neurosci. 2004 Jun;19(12):3317-27. doi: 10.1111/j.0953-816X.2004.03385.x. Eur J Neurosci. 2004. PMID: 15217387
-
Pleiotropic effects of a disrupted K+ channel gene: reduced body weight, impaired motor skill and muscle contraction, but no seizures.Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1533-8. doi: 10.1073/pnas.94.4.1533. Proc Natl Acad Sci U S A. 1997. PMID: 9037088 Free PMC article.
-
Myoclonus epilepsy and ataxia due to potassium channel mutation (MEAK) is caused by heterozygous KCNC1 mutations.Epileptic Disord. 2016 Sep 1;18(S2):135-138. doi: 10.1684/epd.2016.0859. Epileptic Disord. 2016. PMID: 27629860 Review. English.
-
The role of Kv3-type potassium channels in cerebellar physiology and behavior.Cerebellum. 2009 Sep;8(3):323-33. doi: 10.1007/s12311-009-0098-4. Epub 2009 Feb 27. Cerebellum. 2009. PMID: 19247732 Review.
Cited by
-
Precise localization of the voltage-gated potassium channel subunits Kv3.1b and Kv3.3 revealed in the molecular layer of the rat cerebellar cortex by a pre-embedding immunogold method.Histochem Cell Biol. 2010 Oct;134(4):403-9. doi: 10.1007/s00418-010-0742-6. Epub 2010 Sep 21. Histochem Cell Biol. 2010. PMID: 20857303
-
Cerebellar contribution to threat probability in a SCA6 mouse model.Hum Mol Genet. 2022 Nov 10;31(22):3807-3828. doi: 10.1093/hmg/ddac135. Hum Mol Genet. 2022. PMID: 35708512 Free PMC article.
-
Alcohol selectivity of β3-containing GABAA receptors: evidence for a unique extracellular alcohol/imidazobenzodiazepine Ro15-4513 binding site at the α+β- subunit interface in αβ3δ GABAA receptors.Neurochem Res. 2014 Jun;39(6):1118-26. doi: 10.1007/s11064-014-1243-0. Epub 2014 Feb 6. Neurochem Res. 2014. PMID: 24500446 Free PMC article.
-
Encephalopathies with KCNC1 variants: genotype-phenotype-functional correlations.Ann Clin Transl Neurol. 2019 Jul;6(7):1263-1272. doi: 10.1002/acn3.50822. Epub 2019 Jul 1. Ann Clin Transl Neurol. 2019. PMID: 31353855 Free PMC article.
-
A missense mutation in Kcnc3 causes hippocampal learning deficits in mice.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2204901119. doi: 10.1073/pnas.2204901119. Epub 2022 Jul 26. Proc Natl Acad Sci U S A. 2022. PMID: 35881790 Free PMC article.
References
-
- Chan E. PhD thesis. The Rockefeller University; 1997. Regulation and function of Kv3.3.
-
- Espinosa F, Ho CS, McMahon A, Chan E, Heintz N, Burns D, Joho RH. Kv3.1/3.3 double-mutant mice display ataxia and myoclonus. Soc Neurosci Abstr. 2000;26:898.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
