Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal
- PMID: 11517247
- PMCID: PMC6763080
- DOI: 10.1523/JNEUROSCI.21-17-06577.2001
Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal
Abstract
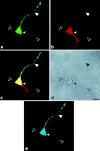
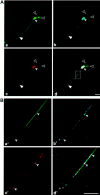
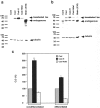
Subcellular mRNA localization, a fundamental mechanism for regulating gene expression, leads to local protein translation that results in the generation of neuronal cell polarity. In this study, we have used P19 embryonic carcinoma cells, which are amenable to transfection, and selection of clonal stable cell lines that are not overexpressing the constructs. We identified the 3' untranslated region (3'UTR) tau axonal localization signal and examined its effect on tau protein localization in nondifferentiated and neuronally differentiated P19 cells. Using GFP-tagged tau constructs combined with in situ hybridization analysis, we demonstrated colocalization of the targeted tau mRNA and its translated protein in the axon and growth cone. Absence of or mutation in the 3'UTR axonal targeting region of tau mRNA resulted in suppression of tau mRNA localization, and both tau mRNA and tau protein remained in the cell body. Swapping between the 3'UTR tau mRNA axonal localization signal and the 3'UTR MAP2 mRNA dendritic targeting signal proved that the localization of the proteins into the axon or dendrites depends on the specific 3'UTR targeting signals. Moreover, the identification of ribosomal proteins in the axon lends further support to the presence of protein synthetic machinery in the axons, a prerequisite for local translation. It is suggested therefore that the P19 cell system can be used to analyze mutations that affect mRNA transport and local translation and that it has the potential of being used to examine the onset of the neuronal differentiation process.
Figures







Similar articles
-
Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules.J Cell Sci. 2002 Oct 1;115(Pt 19):3817-27. doi: 10.1242/jcs.00058. J Cell Sci. 2002. PMID: 12235292
-
A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3' untranslated region AU-rich regulatory element.J Neurochem. 2013 Sep;126(6):792-804. doi: 10.1111/jnc.12266. Epub 2013 Apr 30. J Neurochem. 2013. PMID: 23586486 Free PMC article.
-
The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells.J Neurochem. 2004 May;89(3):613-26. doi: 10.1111/j.1471-4159.2004.02371.x. J Neurochem. 2004. PMID: 15086518
-
Mechanisms of Axonal Sorting of Tau and Influence of the Axon Initial Segment on Tau Cell Polarity.Adv Exp Med Biol. 2019;1184:69-77. doi: 10.1007/978-981-32-9358-8_6. Adv Exp Med Biol. 2019. PMID: 32096029 Review.
-
To localize or not to localize: mRNA fate is in 3'UTR ends.Trends Cell Biol. 2009 Sep;19(9):465-74. doi: 10.1016/j.tcb.2009.06.001. Epub 2009 Aug 26. Trends Cell Biol. 2009. PMID: 19716303 Review.
Cited by
-
Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons.J Neurosci. 2005 Jan 26;25(4):778-91. doi: 10.1523/JNEUROSCI.4235-04.2005. J Neurosci. 2005. PMID: 15673657 Free PMC article.
-
Rehabilitating a brain with Alzheimer's: a proposal.Clin Interv Aging. 2011;6:53-9. doi: 10.2147/CIA.S14008. Epub 2011 Feb 22. Clin Interv Aging. 2011. PMID: 21472092 Free PMC article. Review.
-
Dipeptidyl peptidase 10 (DPP10(789)): a voltage gated potassium channel associated protein is abnormally expressed in Alzheimer's and other neurodegenerative diseases.Biomed Res Int. 2014;2014:209398. doi: 10.1155/2014/209398. Epub 2014 Jun 16. Biomed Res Int. 2014. PMID: 25025038 Free PMC article.
-
A mitochondria cluster at the proximal axon initial segment controls axodendritic TAU trafficking in rodent primary and human iPSC-derived neurons.Cell Mol Life Sci. 2022 Feb 4;79(2):120. doi: 10.1007/s00018-022-04150-3. Cell Mol Life Sci. 2022. PMID: 35119496 Free PMC article.
-
The Role of Microglia in the Spread of Tau: Relevance for Tauopathies.Front Cell Neurosci. 2018 Jul 10;12:172. doi: 10.3389/fncel.2018.00172. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30042659 Free PMC article. Review.
References
-
- Antic D, Keene JD. Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J Cell Sci. 1998;111:183–197. - PubMed
-
- Aronov S, Marx R, Ginzburg I. Identification of 3′ UTR regions implicated in tau mRNA stabilization in neuronal cells. J Mol Neurosci. 1999;12:131–145. - PubMed
-
- Bassell G, Singer RH. mRNA and cytoskeletal filaments. Curr Opin Cell Biol. 1997;9:109–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
