Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294
- PMID: 11509655
- PMCID: PMC87329
- DOI: 10.1128/MCB.21.18.6122-6131.2001
Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294
Abstract
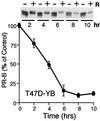
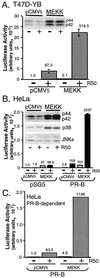
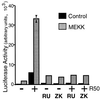
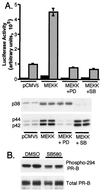
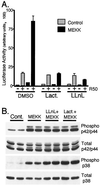
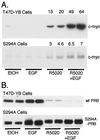
Breast cancers often exhibit elevated expression of tyrosine kinase growth factor receptors; these pathways influence breast cancer cell growth in part by targeting steroid hormone receptors, including progesterone receptors (PR). To mimic activation of molecules downstream of growth factor-initiated signaling pathways, we overexpressed mitogen-activated protein kinase (MAPK; also known as extracellular signal-regulated kinase) kinase kinase 1 (MEKK1) in T47D human breast cancer cells expressing the B isoform of PR. MEKK1 is a strong activator of p42 and p44 MAPKs. MEKK1 expression increased progestin-mediated transcription 8- to 10-fold above normal PR-driven transcription levels. This was dependent on the presence of a progesterone response element and functional PR. PR protein levels were unchanged by MEKK1 alone but were extensively down-regulated by MEKK1 plus the progestin R5020. MEKK1 expression resulted in phosphorylation of PR on Ser294, a MAPK consensus site known to mediate ligand-dependent PR degradation. MEK inhibitors blocked phosphorylation of Ser294 and attenuated PR transcriptional hyperactivity in response to MEKK1 plus R5020; stabilization of PR by inhibition of the 26S proteasome produced similar results. T47D cells stably expressing mutant S294A PR, in which serine 294 is replaced by alanine, fail to undergo ligand-dependent down-regulation and are resistant to MEKK1-plus-R5020-induced transcriptional synergy but respond to progestins alone. Similarly, c-myc protein levels are synergistically increased by epidermal growth factor and R5020 in cells expressing wild-type PR, but not S294A PR. Thus, highly stable mutant PR are functional in response to progestins but are incapable of cross talk with MAPK-driven pathways. These studies demonstrate a paradoxical coupling between steroid receptor down-regulation and transcriptional hyperactivity. They also suggest a link between phosphorylation of PR by MAPKs in response to peptide growth factor signaling and steroid hormone control of breast cancer cell growth.
Figures

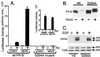
Similar articles
-
Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases.Mol Endocrinol. 2005 Feb;19(2):327-39. doi: 10.1210/me.2004-0306. Epub 2004 Oct 14. Mol Endocrinol. 2005. PMID: 15486045
-
Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth.Steroids. 2007 Feb;72(2):188-201. doi: 10.1016/j.steroids.2006.11.009. Epub 2006 Dec 14. Steroids. 2007. PMID: 17173941 Free PMC article.
-
Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors.Mol Endocrinol. 2003 Apr;17(4):628-42. doi: 10.1210/me.2002-0378. Epub 2003 Jan 9. Mol Endocrinol. 2003. PMID: 12554776
-
MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):147-57. doi: 10.1016/s0960-0760(03)00221-8. J Steroid Biochem Mol Biol. 2003. PMID: 12943699 Review.
-
Progesterone receptor action: translating studies in breast cancer models to clinical insights.Adv Exp Med Biol. 2008;630:94-111. Adv Exp Med Biol. 2008. PMID: 18637487 Review.
Cited by
-
Phosphorylation: a fundamental regulator of steroid receptor action.Trends Endocrinol Metab. 2013 Oct;24(10):515-24. doi: 10.1016/j.tem.2013.05.008. Epub 2013 Jul 6. Trends Endocrinol Metab. 2013. PMID: 23838532 Free PMC article. Review.
-
Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1.J Biol Chem. 2015 Sep 4;290(36):21876-89. doi: 10.1074/jbc.M115.657957. Epub 2015 Jul 22. J Biol Chem. 2015. PMID: 26203193 Free PMC article.
-
Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2.Mol Cell Biol. 2004 Dec;24(24):10542-57. doi: 10.1128/MCB.24.24.10542-10557.2004. Mol Cell Biol. 2004. PMID: 15572662 Free PMC article.
-
Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line.PLoS One. 2012;7(9):e45993. doi: 10.1371/journal.pone.0045993. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029355 Free PMC article.
-
p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization.Mol Endocrinol. 2011 Oct;25(10):1710-24. doi: 10.1210/me.2011-1042. Epub 2011 Aug 4. Mol Endocrinol. 2011. PMID: 21816898 Free PMC article.
References
-
- Aronica S M, Katzenellenbogen B S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. - PubMed
-
- Beck C A, Weigel N L, Edwards D P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
