A protein interaction map for cell polarity development
- PMID: 11489916
- PMCID: PMC2196425
- DOI: 10.1083/jcb.200104057
A protein interaction map for cell polarity development
Abstract
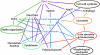
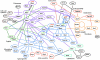
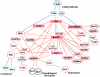
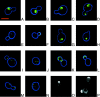
Many genes required for cell polarity development in budding yeast have been identified and arranged into a functional hierarchy. Core elements of the hierarchy are widely conserved, underlying cell polarity development in diverse eukaryotes. To enumerate more fully the protein-protein interactions that mediate cell polarity development, and to uncover novel mechanisms that coordinate the numerous events involved, we carried out a large-scale two-hybrid experiment. 68 Gal4 DNA binding domain fusions of yeast proteins associated with the actin cytoskeleton, septins, the secretory apparatus, and Rho-type GTPases were used to screen an array of yeast transformants that express approximately 90% of the predicted Saccharomyces cerevisiae open reading frames as Gal4 activation domain fusions. 191 protein-protein interactions were detected, of which 128 had not been described previously. 44 interactions implicated 20 previously uncharacterized proteins in cell polarity development. Further insights into possible roles of 13 of these proteins were revealed by their multiple two-hybrid interactions and by subcellular localization. Included in the interaction network were associations of Cdc42 and Rho1 pathways with proteins involved in exocytosis, septin organization, actin assembly, microtubule organization, autophagy, cytokinesis, and cell wall synthesis. Other interactions suggested direct connections between Rho1- and Cdc42-regulated pathways; the secretory apparatus and regulators of polarity establishment; actin assembly and the morphogenesis checkpoint; and the exocytic and endocytic machinery. In total, a network of interactions that provide an integrated response of signaling proteins, the cytoskeleton, and organelles to the spatial cues that direct polarity development was revealed.
Figures


Similar articles
-
Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae.Mol Cell Biol. 2001 Feb;21(3):827-39. doi: 10.1128/MCB.21.3.827-839.2001. Mol Cell Biol. 2001. PMID: 11154270 Free PMC article.
-
Cell cycle control of septin ring dynamics in the budding yeast.Microbiology (Reading). 2001 Jun;147(Pt 6):1437-1450. doi: 10.1099/00221287-147-6-1437. Microbiology (Reading). 2001. PMID: 11390675
-
Interaction between a Ras and a Rho GTPase couples selection of a growth site to the development of cell polarity in yeast.Mol Biol Cell. 2003 Dec;14(12):4958-70. doi: 10.1091/mbc.e03-06-0426. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960420 Free PMC article.
-
Polarity and division site specification in yeast.Curr Opin Microbiol. 1998 Dec;1(6):678-86. doi: 10.1016/s1369-5274(98)80115-x. Curr Opin Microbiol. 1998. PMID: 10066541 Review.
-
Symmetry breaking in the life cycle of the budding yeast.Cold Spring Harb Perspect Biol. 2009 Sep;1(3):a003384. doi: 10.1101/cshperspect.a003384. Cold Spring Harb Perspect Biol. 2009. PMID: 20066112 Free PMC article. Review.
Cited by
-
The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans.Mol Microbiol. 2012 Apr;84(1):166-80. doi: 10.1111/j.1365-2958.2012.08017.x. Epub 2012 Mar 5. Mol Microbiol. 2012. PMID: 22384976 Free PMC article.
-
Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy.Mol Biol Cell. 2005 May;16(5):2544-53. doi: 10.1091/mbc.e04-08-0669. Epub 2005 Mar 2. Mol Biol Cell. 2005. PMID: 15743910 Free PMC article.
-
Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae.Mol Biol Cell. 2004 May;15(5):2189-204. doi: 10.1091/mbc.e03-07-0479. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004240 Free PMC article.
-
Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore.Genes Dev. 2002 Jan 1;16(1):101-13. doi: 10.1101/gad.949302. Genes Dev. 2002. PMID: 11782448 Free PMC article.
-
A drug-sensitive genetic network masks fungi from the immune system.PLoS Pathog. 2006 Apr;2(4):e35. doi: 10.1371/journal.ppat.0020035. Epub 2006 Apr 28. PLoS Pathog. 2006. PMID: 16652171 Free PMC article.
References
-
- Albert, S., and D. Gallwitz. 1999. Two new members of a family of Ypt/Rab GTP-ase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem. 274:33186–33189. - PubMed
-
- Albert, S., and D. Gallwitz. 2000. Msb4p, a protein involved in Cdc42p-dependent organization of the actin cytoskeleton, is a Ypt/Rab-specific GAP. Biol. Chem. 381:453–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM035370/GM/NIGMS NIH HHS/United States
- R01 GM50399/GM/NIGMS NIH HHS/United States
- R01 GM54712/GM/NIGMS NIH HHS/United States
- R01 GM059216/GM/NIGMS NIH HHS/United States
- R01 GM35370/GM/NIGMS NIH HHS/United States
- R01 GM054712/GM/NIGMS NIH HHS/United States
- R37 GM031006/GM/NIGMS NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
- P41 RR11823/RR/NCRR NIH HHS/United States
- R01 GM59216/GM/NIGMS NIH HHS/United States
- R01 GM31006/GM/NIGMS NIH HHS/United States
- R01 GM035370/GM/NIGMS NIH HHS/United States
- R01 GM050399/GM/NIGMS NIH HHS/United States
- R01 GM031006/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

