Structure-based mutational analysis of the hepatitis C virus NS3 helicase
- PMID: 11483774
- PMCID: PMC115073
- DOI: 10.1128/jvi.75.17.8289-8297.2001
Structure-based mutational analysis of the hepatitis C virus NS3 helicase
Abstract
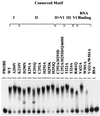
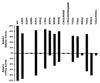
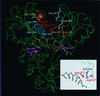
The carboxyl terminus of the hepatitis C virus (HCV) nonstructural protein 3 (NS3) possesses ATP-dependent RNA helicase activity. Based on the conserved sequence motifs and the crystal structures of the helicase domain, 17 mutants of the HCV NS3 helicase were generated. The ATP hydrolysis, RNA binding, and RNA unwinding activities of the mutant proteins were examined in vitro to determine the functional role of the mutated residues. The data revealed that Lys-210 in the Walker A motif and Asp-290, Glu-291, and His-293 in the Walker B motif were crucial to ATPase activity and that Thr-322 and Thr-324 in motif III and Arg-461 in motif VI significantly influenced ATPase activity. When the pairing between His-293 and Gln-460, referred to as gatekeepers, was replaced with the Asp-293/His-460 pair, which makes the NS3 helicase more like the DEAD helicase subgroup, ATPase activity was not restored. It thus indicated that the whole microenvironment surrounding the gatekeepers, rather than the residues per se, was important to the enzymatic activities. Arg-461 and Trp-501 are important residues for RNA binding, while Val-432 may only play a coadjutant role. The data demonstrated that RNA helicase activity was possibly abolished by the loss of ATPase activity or by reduced RNA binding activity. Nevertheless, a low threshold level of ATPase activity was found sufficient for helicase activity. Results in this study provide a valuable reference for efforts under way to develop anti-HCV therapeutic drugs targeting NS3.
Figures


Similar articles
-
Solution structure and backbone dynamics of an engineered arginine-rich subdomain 2 of the hepatitis C virus NS3 RNA helicase.J Mol Biol. 2001 Nov 30;314(3):543-61. doi: 10.1006/jmbi.2001.5146. J Mol Biol. 2001. PMID: 11846566
-
Role of the DExH motif of the Japanese encephalitis virus and hepatitis C virus NS3 proteins in the ATPase and RNA helicase activities.Virology. 2000 Aug 1;273(2):316-24. doi: 10.1006/viro.2000.0417. Virology. 2000. PMID: 10915602
-
Hepatitis C virus NS3 RNA helicase activity is modulated by the two domains of NS3 and NS4A.Biochem Biophys Res Commun. 2004 Apr 23;317(1):211-7. doi: 10.1016/j.bbrc.2004.03.032. Biochem Biophys Res Commun. 2004. PMID: 15047170
-
Structure and function of hepatitis C virus NS3 helicase.Curr Top Microbiol Immunol. 2000;242:171-96. doi: 10.1007/978-3-642-59605-6_9. Curr Top Microbiol Immunol. 2000. PMID: 10592661 Review.
-
Conserved motifs in the flavivirus NS3 RNA helicase enzyme.Wiley Interdiscip Rev RNA. 2022 Mar;13(2):e1688. doi: 10.1002/wrna.1688. Epub 2021 Sep 2. Wiley Interdiscip Rev RNA. 2022. PMID: 34472205 Free PMC article. Review.
Cited by
-
Electrostatic analysis of the hepatitis C virus NS3 helicase reveals both active and allosteric site locations.Nucleic Acids Res. 2004 Oct 12;32(18):5519-28. doi: 10.1093/nar/gkh891. Print 2004. Nucleic Acids Res. 2004. PMID: 15479787 Free PMC article.
-
Two novel conserved motifs in the hepatitis C virus NS3 protein critical for helicase action.J Biol Chem. 2003 Nov 7;278(45):44514-24. doi: 10.1074/jbc.M306444200. Epub 2003 Aug 27. J Biol Chem. 2003. PMID: 12944414 Free PMC article.
-
Zika virus NS3 drives the assembly of a viroplasm-like structure.bioRxiv [Preprint]. 2024 Sep 16:2024.09.16.613201. doi: 10.1101/2024.09.16.613201. bioRxiv. 2024. PMID: 39345390 Free PMC article. Preprint.
-
Inhibition of hepatitis C virus nonstructural protein, helicase activity, and viral replication by a recombinant human antibody clone.Am J Pathol. 2004 Oct;165(4):1163-73. doi: 10.1016/S0002-9440(10)63377-9. Am J Pathol. 2004. PMID: 15466383 Free PMC article.
-
Structurally conserved amino Acid w501 is required for RNA helicase activity but is not essential for DNA helicase activity of hepatitis C virus NS3 protein.J Virol. 2003 Jan;77(1):571-82. doi: 10.1128/jvi.77.1.571-582.2003. J Virol. 2003. PMID: 12477861 Free PMC article.
References
-
- Black M E, Hruby D E. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J Biol Chem. 1992;267:6801–6806. - PubMed
-
- Brillanti S, Garson J, Foli M, Whitby K, Deaville R, Masci C, Miglioli M, Barbara L. A pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa-resistant chronic hepatitis C. Gastroenterology. 1994;107:812–817. - PubMed
-
- Brillanti S, Miglioli M, Barbara L. Combination antiviral therapy with ribavirin and interferon alfa in interferon alfa relapsers and non-responders: Italian experience. J Hepatol. 1995;23:13–15. - PubMed
-
- Chamberlain R W, Adams N, Saeed A A, Simmonds P, Elliott R M. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J Gen Virol. 1997;78:1341–1347. - PubMed
-
- Chemello L, Cavalletto L, Bernardinello E, Guido M, Pontisso P, Alberti A. The effect of interferon alfa and ribavirin combination therapy in naive patients with chronic hepatitis C. J Hepatol. 1995;23:8–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

