Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains
- PMID: 11483736
- PMCID: PMC115035
- DOI: 10.1128/jvi.75.17.7913-7924.2001
Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains
Abstract
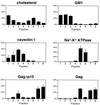
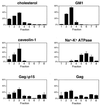
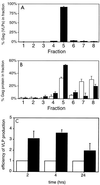
The Gag polyprotein of human immunodeficiency virus type 1 (HIV-1) organizes the assembly of nascent virions at the plasma membrane of infected cells. Here we demonstrate that a population of Gag is present in distinct raft-like membrane microdomains that we have termed "barges." Barges have a higher density than standard rafts, most likely due to the presence of oligomeric Gag-Gag assembly complexes. The regions of the Gag protein responsible for barge targeting were mapped by examining the flotation behavior of wild-type and mutant proteins on Optiprep density gradients. N-myristoylation of Gag was necessary for association with barges. Removal of the NC and p6 domains shifted much of the Gag from barges into typical raft fractions. These data are consistent with a model in which multimerization of myristoylated Gag proteins drives association of Gag oligomers into raft-like barges. The functional significance of barge association was revealed by several lines of evidence. First, Gag isolated from virus-like particles was almost entirely localized in barges. Moreover, a comparison of wild-type Gag with Fyn(10)Gag, a chimeric protein containing the N-terminal sequence of Fyn, revealed that Fyn(10)Gag exhibited increased affinity for barges and a two- to fourfold increase in particle production. These results imply that association of Gag with raft-like barge membrane microdomains plays an important role in the HIV-1 assembly process.
Figures






Similar articles
-
Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100.J Virol. 2003 Apr;77(8):4805-17. doi: 10.1128/jvi.77.8.4805-4817.2003. J Virol. 2003. PMID: 12663787 Free PMC article.
-
Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts.J Virol. 2003 Feb;77(3):1916-26. doi: 10.1128/jvi.77.3.1916-1926.2003. J Virol. 2003. PMID: 12525626 Free PMC article.
-
Defect of human immunodeficiency virus type 2 Gag assembly in Saccharomyces cerevisiae.J Virol. 2007 Sep;81(18):9911-21. doi: 10.1128/JVI.00027-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609278 Free PMC article.
-
Role of HIV-1 Gag domains in viral assembly.Biochim Biophys Acta. 2003 Jul 11;1614(1):62-72. doi: 10.1016/s0005-2736(03)00163-9. Biochim Biophys Acta. 2003. PMID: 12873766 Review.
-
[Subcellular locations at which HIV-1 assembles].Uirusu. 2007 Jun;57(1):9-18. doi: 10.2222/jsv.57.9. Uirusu. 2007. PMID: 18040150 Review. Japanese.
Cited by
-
Basic residues in the matrix domain and multimerization target murine leukemia virus Gag to the virological synapse.J Virol. 2013 Jun;87(12):7113-26. doi: 10.1128/JVI.03263-12. Epub 2013 Apr 24. J Virol. 2013. PMID: 23616653 Free PMC article.
-
Posttranslational modifications and secretion efficiency of immunogenic hepatitis B virus L protein deletion variants.Virol J. 2013 Feb 25;10:63. doi: 10.1186/1743-422X-10-63. Virol J. 2013. PMID: 23442390 Free PMC article.
-
Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1.J Cell Biol. 2006 Jun 5;173(5):795-807. doi: 10.1083/jcb.200508165. Epub 2006 May 30. J Cell Biol. 2006. PMID: 16735575 Free PMC article.
-
HIV-1 assembly in macrophages.Retrovirology. 2010 Apr 7;7:29. doi: 10.1186/1742-4690-7-29. Retrovirology. 2010. PMID: 20374631 Free PMC article. Review.
-
Amphotropic murine leukaemia virus envelope protein is associated with cholesterol-rich microdomains.Virol J. 2005 Apr 19;2:36. doi: 10.1186/1743-422X-2-36. Virol J. 2005. PMID: 15840168 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

