Efficient chromosomal transposition of a Tc1/mariner- like transposon Sleeping Beauty in mice
- PMID: 11481482
- PMCID: PMC55396
- DOI: 10.1073/pnas.161071798
Efficient chromosomal transposition of a Tc1/mariner- like transposon Sleeping Beauty in mice
Abstract
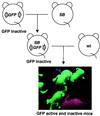
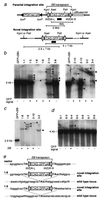
The presence of mouse embryonic stem (ES) cells makes the mouse a powerful model organism for reverse genetics, gene function study through mutagenesis of specific genes. In contrast, forward genetics, identification of mutated genes responsible for specific phenotypes, has an advantage to uncover novel pathways and unknown genes because no a priori assumptions are made about the mutated genes. However, it has been hampered in mice because of the lack of a system in which a large-scale mutagenesis and subsequent isolation of mutated genes can be performed efficiently. Here, we demonstrate the efficient chromosomal transposition of a Tc1/mariner-like transposon, Sleeping Beauty, in mice. This system allows germ-line mutagenesis in vivo and will facilitate certain aspects of phenotype-driven genetic screening in mice.
Figures



Similar articles
-
Insertional mutagenesis of the mouse germline with Sleeping Beauty transposition.Methods Mol Biol. 2008;435:109-25. doi: 10.1007/978-1-59745-232-8_8. Methods Mol Biol. 2008. PMID: 18370071
-
Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system.Nat Methods. 2005 Oct;2(10):763-9. doi: 10.1038/nmeth795. Nat Methods. 2005. PMID: 16179923
-
Transposable elements for transgenesis and insertional mutagenesis in vertebrates: a contemporary review of experimental strategies.Methods Mol Biol. 2004;260:255-76. doi: 10.1385/1-59259-755-6:255. Methods Mol Biol. 2004. PMID: 15020812
-
Insertional engineering of chromosomes with Sleeping Beauty transposition: an overview.Methods Mol Biol. 2011;738:69-85. doi: 10.1007/978-1-61779-099-7_5. Methods Mol Biol. 2011. PMID: 21431720 Review.
-
Generation of radiation-induced deletion complexes in the mouse genome using embryonic stem cells.Methods. 1997 Dec;13(4):409-21. doi: 10.1006/meth.1997.0547. Methods. 1997. PMID: 9480785 Review.
Cited by
-
Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system.Mamm Genome. 2007 May;18(5):338-46. doi: 10.1007/s00335-007-9025-5. Epub 2007 Jun 8. Mamm Genome. 2007. PMID: 17557177
-
The medaka fish Tol2 transposable element can undergo excision in human and mouse cells.J Hum Genet. 2003;48(5):231-235. doi: 10.1007/s10038-003-0016-4. Epub 2003 Mar 28. J Hum Genet. 2003. PMID: 12768440
-
Technology transfer from worms and flies to vertebrates: transposition-based genome manipulations and their future perspectives.Genome Biol. 2007;8 Suppl 1(Suppl 1):S1. doi: 10.1186/gb-2007-8-s1-s1. Genome Biol. 2007. PMID: 18047686 Free PMC article. Review.
-
Insertional mutagenesis strategies in zebrafish.Genome Biol. 2007;8 Suppl 1(Suppl 1):S9. doi: 10.1186/gb-2007-8-s1-s9. Genome Biol. 2007. PMID: 18047701 Free PMC article. Review.
-
Trans-kingdom transposition of the maize dissociation element.Genetics. 2006 Nov;174(3):1095-104. doi: 10.1534/genetics.106.061184. Epub 2006 Sep 1. Genetics. 2006. PMID: 16951067 Free PMC article.
References
-
- Capecchi M R. Science. 1989;244:1288–1292. - PubMed
-
- Nolan P M, Peters J, Strivens M, Rogers D, Hagan J, Spurr N, Gray I C, Vizor L, Brooker D, Whitenhill E, et al. Nat Genet. 2000;25:440–443. - PubMed
-
- de Angelis M H, Flaswinkel H, Fuchs H, Rathkolb B, Soewarto D, Marschall S, Heffner S, Pargent W, Wuensch K, Jung M, et al. Nat Genet. 2000;25:444–447. - PubMed
-
- Bellen H J, O'Kane C J, Wilson C, Grossniklaus U, Pearson R K, Gehring W J. Genes Dev. 1989;3:1288–1300. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

