Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism
- PMID: 11463816
- PMCID: PMC87256
- DOI: 10.1128/MCB.21.16.5321-5331.2001
Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism
Abstract
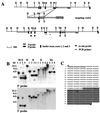
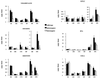
We have created a transgenic mouse with a hypomorphic allele of the vesicular monoamine transporter 2 (Vmat2) gene by gene targeting. These mice (KA1) have profound changes in monoamine metabolism and function and survive into adulthood. Specifically, these animals express very low levels of VMAT2, an endogenous protein which sequesters monoamines intracellularly into vesicles, a process that, in addition to being important in normal transmission, may also act to keep intracellular levels of the monoamine neurotransmitters below potentially toxic thresholds. Homozygous mice show large reductions in brain tissue monoamines, motor impairments, enhanced sensitivity to dopamine agonism, and changes in the chemical neuroanatomy of the striatum that are consistent with alterations in the balance of the striatonigral (direct) and striatopallidal (indirect) pathways. The VMAT2-deficient KA1 mice are also more vulnerable to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in terms of nigral dopamine cell death. We suggest that the mice may be of value in examining, long term, the insidious damaging consequences of abnormal intracellular handling of monoamines. On the basis of our current findings, the mice are likely to prove of immediate interest to aspects of the symptomatology of parkinsonism. They may also, however, be of use in probing other aspects of monoaminergic function and dysfunction in the brain, the latter making important contributions to the pathogenesis of schizophrenia and addiction.
Figures





Similar articles
-
Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata.J Pharmacol Exp Ther. 2000 May;293(2):336-42. J Pharmacol Exp Ther. 2000. PMID: 10773000
-
The role of membrane and vesicular monoamine transporters in the neurotoxic and hypothermic effects of 1-methyl-4-(2'-aminophenyl)-1,2,3,6-tetrahydropyridine (2'-NH(2)-MPTP).Mol Pharmacol. 2004 Sep;66(3):718-27. doi: 10.1124/mol.66.3.. Mol Pharmacol. 2004. PMID: 15322265
-
Rapid and differential losses of in vivo dopamine transporter (DAT) and vesicular monoamine transporter (VMAT2) radioligand binding in MPTP-treated mice.Synapse. 2000 Mar 15;35(4):250-5. doi: 10.1002/(SICI)1098-2396(20000315)35:4<250::AID-SYN2>3.0.CO;2-S. Synapse. 2000. PMID: 10657034
-
In vivo imaging of the vesicular acetylcholine transporter and the vesicular monoamine transporter.FASEB J. 2000 Dec;14(15):2401-13. doi: 10.1096/fj.00-0204rev. FASEB J. 2000. PMID: 11099458 Review.
-
The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins.FASEB J. 2000 Dec;14(15):2459-65. doi: 10.1096/fj.00-0205rev. FASEB J. 2000. PMID: 11099463 Review.
Cited by
-
Increased endogenous dopamine prevents myopia in mice.Exp Eye Res. 2020 Apr;193:107956. doi: 10.1016/j.exer.2020.107956. Epub 2020 Feb 4. Exp Eye Res. 2020. PMID: 32032629 Free PMC article.
-
Genetically engineered mouse models of Parkinson's disease.Brain Res Bull. 2012 May 1;88(1):13-32. doi: 10.1016/j.brainresbull.2011.07.019. Epub 2011 Aug 3. Brain Res Bull. 2012. PMID: 21839151 Free PMC article. Review.
-
Assessing Vesicular Monoamine Transport and Toxicity Using Fluorescent False Neurotransmitters.Chem Res Toxicol. 2021 May 17;34(5):1256-1264. doi: 10.1021/acs.chemrestox.0c00380. Epub 2020 Dec 30. Chem Res Toxicol. 2021. PMID: 33378168 Free PMC article.
-
Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson's disease.Neurobiol Aging. 2013 Jun;34(6):1712.e9-13. doi: 10.1016/j.neurobiolaging.2012.12.020. Epub 2013 Jan 28. Neurobiol Aging. 2013. PMID: 23369548 Free PMC article.
-
Vesicular monoamine transporter 2 mRNA levels are reduced in platelets from patients with Parkinson's disease.J Neural Transm (Vienna). 2010 Sep;117(9):1093-8. doi: 10.1007/s00702-010-0446-z. Epub 2010 Jul 28. J Neural Transm (Vienna). 2010. PMID: 20665056
References
-
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. - PubMed
-
- Bergman H, Wichmann T, DeLong M R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. - PubMed
-
- Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. - PubMed
-
- Chase T N, Oh J D, Blanchet P J. Neostriatal mechanisms in Parkinson's disease. Neurology. 1998;51:30–35. - PubMed
-
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. 1. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;62:232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
