Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells
- PMID: 11462053
- PMCID: PMC115016
- DOI: 10.1128/JVI.75.16.7769-7773.2001
Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells
Abstract
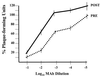
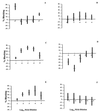
The specific mechanisms by which antibodies neutralize flavivirus infectivity are not completely understood. To study these mechanisms in more detail, we analyzed the ability of a well-defined set of anti-dengue (DEN) virus E-glycoprotein-specific monoclonal antibodies (MAbs) to block virus adsorption to Vero cells. In contrast to previous studies, the binding sites of these MAbs were localized to one of three structural domains (I, II, and III) in the E glycoprotein. The results indicate that most MAbs that neutralize virus infectivity do so, at least in part, by the blocking of virus adsorption. However, MAbs specific for domain III were the strongest blockers of virus adsorption. These results extend our understanding of the structure-function relationships in the E glycoprotein of DEN virus and provide the first direct evidence that domain III encodes the primary flavivirus receptor-binding motif.
Figures

Similar articles
-
Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica.Virology. 1998 Jul 5;246(2):317-28. doi: 10.1006/viro.1998.9200. Virology. 1998. PMID: 9657950
-
Epitope mapping of dengue 1 virus E glycoprotein using monoclonal antibodies.Arch Virol. 1995;140(7):1257-73. doi: 10.1007/BF01322751. Arch Virol. 1995. PMID: 7646356
-
[Recombinant envelope glycoprotein domain III of dengue virus inhibit virus infection].Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008 Jun;22(3):177-9. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008. PMID: 19031695 Chinese.
-
Survival of mice immunized with monoclonal antibodies against glycoprotein E of Japanese encephalitis virus before or after infection with Japanese encephalitis, West Nile, and Dengue viruses.Acta Virol. 2008;52(4):219-24. Acta Virol. 2008. PMID: 19143477
-
Analysis of the steps involved in Dengue virus entry into host cells.Virology. 1999 Apr 25;257(1):156-67. doi: 10.1006/viro.1999.9633. Virology. 1999. PMID: 10208929
Cited by
-
DENV-2 subunit proteins fused to CR2 receptor-binding domain (P28)-induces specific and neutralizing antibodies to the Dengue virus in mice.Hum Vaccin Immunother. 2013 Nov;9(11):2326-35. doi: 10.4161/hv.25673. Epub 2013 Jul 23. Hum Vaccin Immunother. 2013. PMID: 23880886 Free PMC article.
-
Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity.Microbiol Mol Biol Rev. 2016 Oct 26;80(4):989-1010. doi: 10.1128/MMBR.00024-15. Print 2016 Dec. Microbiol Mol Biol Rev. 2016. PMID: 27784796 Free PMC article. Review.
-
Systematic Bioinformatic Approach for Prediction of Linear B-Cell Epitopes on Dengue E and prM Protein.Adv Bioinformatics. 2016;2016:1373157. doi: 10.1155/2016/1373157. Epub 2016 Sep 1. Adv Bioinformatics. 2016. PMID: 27688753 Free PMC article.
-
A multiplex ELISA-based protein array for screening diagnostic antigens and diagnosis of Flaviviridae infection.Eur J Clin Microbiol Infect Dis. 2015 Jul;34(7):1327-36. doi: 10.1007/s10096-015-2353-6. Epub 2015 Mar 22. Eur J Clin Microbiol Infect Dis. 2015. PMID: 25796511
-
Direct Analysis of Mitochondrial Damage Caused by Misfolded/Destabilized Proteins.Int J Mol Sci. 2022 Aug 31;23(17):9881. doi: 10.3390/ijms23179881. Int J Mol Sci. 2022. PMID: 36077279 Free PMC article.
References
-
- Anderson R, King A D, Innis B L. Correlation of E protein binding with cell susceptibility to dengue 4 virus infection. J Gen Virol. 1992;73:2155–2159. - PubMed
-
- Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

