IL-7 is critical for homeostatic proliferation and survival of naive T cells
- PMID: 11447288
- PMCID: PMC37504
- DOI: 10.1073/pnas.161126098
IL-7 is critical for homeostatic proliferation and survival of naive T cells
Abstract
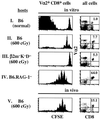
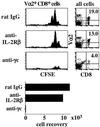
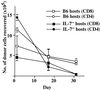
In T cell-deficient conditions, naive T cells undergo spontaneous "homeostatic" proliferation in response to contact with self-MHC/peptide ligands. With the aid of an in vitro system, we show here that homeostatic proliferation is also cytokine-dependent. The cytokines IL-4, IL-7, and IL-15 enhanced homeostatic proliferation of naive T cells in vitro. Of these cytokines, only IL-7 was found to be critical; thus, naive T cells underwent homeostatic proliferation in IL-4(-) and IL-15(-) hosts but proliferated minimally in IL-7(-) hosts. In addition to homeostatic proliferation, the prolonged survival of naive T cells requires IL-7. Thus, naïve T cells disappeared gradually over a 1-month period upon adoptive transfer into IL-7(-) hosts. These findings indicate that naive T cells depend on IL-7 for survival and homeostatic proliferation.
Figures




Similar articles
-
Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo.Nat Immunol. 2000 Nov;1(5):426-32. doi: 10.1038/80868. Nat Immunol. 2000. PMID: 11062503
-
Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells.J Exp Med. 2002 Jun 17;195(12):1523-32. doi: 10.1084/jem.20020066. J Exp Med. 2002. PMID: 12070280 Free PMC article.
-
Cytokine deprivation of naive CD8+ T cells promotes minimal cell cycling but maximal cytokine synthesis and autonomous proliferation subsequently: a mechanism of self-regulation.J Immunol. 1999 Sep 1;163(5):2443-51. J Immunol. 1999. PMID: 10452979
-
gamma(c) cytokines provide multiple homeostatic signals to naive CD4(+) T cells.Eur J Immunol. 2007 Sep;37(9):2606-16. doi: 10.1002/eji.200737234. Eur J Immunol. 2007. PMID: 17683114
-
Generation and maintenance of memory CD4(+) T Cells.Curr Opin Immunol. 2009 Apr;21(2):167-72. doi: 10.1016/j.coi.2009.02.005. Epub 2009 Mar 11. Curr Opin Immunol. 2009. PMID: 19282163 Free PMC article. Review.
Cited by
-
A short CD3/CD28 costimulation combined with IL-21 enhance the generation of human memory stem T cells for adoptive immunotherapy.J Transl Med. 2016 Jul 19;14(1):214. doi: 10.1186/s12967-016-0973-y. J Transl Med. 2016. PMID: 27435312 Free PMC article.
-
Effect of IL-7 and IL-15 on T cell phenotype in myelodysplastic syndromes.Oncotarget. 2016 May 10;7(19):27479-88. doi: 10.18632/oncotarget.8459. Oncotarget. 2016. PMID: 27036031 Free PMC article.
-
SILAC-based quantitative proteomics to investigate the eicosanoid associated inflammatory response in activated macrophages.J Inflamm (Lond). 2022 Sep 1;19(1):12. doi: 10.1186/s12950-022-00309-8. J Inflamm (Lond). 2022. PMID: 36050729 Free PMC article.
-
Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite.Vaccines (Basel). 2021 Mar 18;9(3):277. doi: 10.3390/vaccines9030277. Vaccines (Basel). 2021. PMID: 33803752 Free PMC article.
-
Partial recovery of senescence and differentiation disturbances in CD8+ T cell effector-memory cells in HIV-1 infection after initiation of anti-retroviral treatment.Clin Exp Immunol. 2016 Nov;186(2):227-238. doi: 10.1111/cei.12837. Epub 2016 Aug 23. Clin Exp Immunol. 2016. PMID: 27377704 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

