Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo
- PMID: 11425874
- PMCID: PMC2150725
- DOI: 10.1083/jcb.153.7.1441
Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo
Abstract
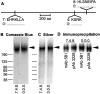
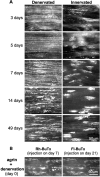
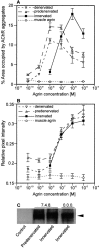
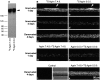
Aggregation of acetylcholine receptors (AChRs) in muscle fibers by nerve-derived agrin plays a key role in the formation of neuromuscular junctions. So far, the effects of agrin on muscle fibers have been studied in culture systems, transgenic animals, and in animals injected with agrin--cDNA constructs. We have applied purified recombinant chick neural and muscle agrin to rat soleus muscle in vivo and obtained the following results. Both neural and muscle agrin bind uniformly to the surface of innervated and denervated muscle fibers along their entire length. Neural agrin causes a dose-dependent appearance of AChR aggregates, which persist > or = 7 wk after a single application. Muscle agrin does not cluster AChRs and at 10 times the concentration of neural agrin does not reduce binding or AChR-aggregating activity of neural agrin. Electrical muscle activity affects the stability of agrin binding and the number, size, and spatial distribution of the neural agrin--induced AChR aggregates. Injected agrin is recovered from the muscles together with laminin and both proteins coimmunoprecipitate, indicating that agrin binds to laminin in vivo. Thus, the present approach provides a novel, simple, and efficient method for studying the effects of agrin on muscle under controlled conditions in vivo.
Figures




Comment in
-
Shaping membrane architecture: agrins in and out of the synapse.J Cell Biol. 2001 Jun 25;153(7):F39-42. doi: 10.1083/jcb.153.7.f39. J Cell Biol. 2001. PMID: 11425880 Free PMC article. No abstract available.
Similar articles
-
Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers.J Cell Biol. 2001 Jun 25;153(7):1453-63. doi: 10.1083/jcb.153.7.1453. J Cell Biol. 2001. PMID: 11425875 Free PMC article.
-
Tyrosine phosphatases such as SHP-2 act in a balance with Src-family kinases in stabilization of postsynaptic clusters of acetylcholine receptors.BMC Neurosci. 2007 Jul 2;8:46. doi: 10.1186/1471-2202-8-46. BMC Neurosci. 2007. PMID: 17605785 Free PMC article.
-
Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers.Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9924-9. doi: 10.1073/pnas.171539698. Epub 2001 Aug 7. Proc Natl Acad Sci U S A. 2001. PMID: 11493710 Free PMC article.
-
What controls the position, number, size, and distribution of neuromuscular junctions on rat muscle fibers?J Neurocytol. 2003 Jun-Sep;32(5-8):835-48. doi: 10.1023/B:NEUR.0000020627.18156.b1. J Neurocytol. 2003. PMID: 15034271 Review.
-
Development of the neuromuscular junction.Cell Tissue Res. 2006 Nov;326(2):263-71. doi: 10.1007/s00441-006-0237-x. Epub 2006 Jul 4. Cell Tissue Res. 2006. PMID: 16819627 Review.
Cited by
-
A conserved RNA switch for acetylcholine receptor clustering at neuromuscular junctions in chordates.bioRxiv [Preprint]. 2024 Jul 6:2024.07.05.602308. doi: 10.1101/2024.07.05.602308. bioRxiv. 2024. PMID: 39005407 Free PMC article. Preprint.
-
Structural basis of agrin-LRP4-MuSK signaling.Genes Dev. 2012 Feb 1;26(3):247-58. doi: 10.1101/gad.180885.111. Genes Dev. 2012. PMID: 22302937 Free PMC article.
-
Modulation of the Acetylcholine Receptor Clustering Pathway Improves Neuromuscular Junction Structure and Muscle Strength in a Mouse Model of Congenital Myasthenic Syndrome.Front Mol Neurosci. 2020 Dec 17;13:594220. doi: 10.3389/fnmol.2020.594220. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33390901 Free PMC article.
-
Activation of the Wnt/β-catenin signaling cascade after traumatic nerve injury.Neuroscience. 2015 May 21;294:101-8. doi: 10.1016/j.neuroscience.2015.02.049. Epub 2015 Mar 3. Neuroscience. 2015. PMID: 25743255 Free PMC article.
-
Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages.J Cell Biol. 2007 Mar 26;176(7):979-93. doi: 10.1083/jcb.200611152. J Cell Biol. 2007. PMID: 17389231 Free PMC article.
References
-
- Bork P., Downing A.K., Kieffer B., Campbell I.D. Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 1996;29:119–167. - PubMed
-
- Chiu A.Y., Sanes J.R. Development of basal lamina in synaptic and extrasynaptic portions of embryonic rat muscle. Dev. Biol. 1984;103:456–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

