Homotypic fusion of immature secretory granules during maturation requires syntaxin 6
- PMID: 11408578
- PMCID: PMC37334
- DOI: 10.1091/mbc.12.6.1699
Homotypic fusion of immature secretory granules during maturation requires syntaxin 6
Abstract
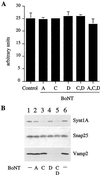
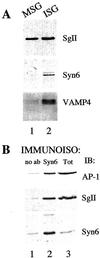
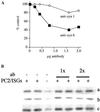
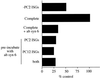
Homotypic fusion of immature secretory granules (ISGs) gives rise to mature secretory granules (MSGs), the storage compartment in endocrine and neuroendocrine cells for hormones and neuropeptides. With the use of a cell-free fusion assay, we investigated which soluble N-ethylmaleimide-sensitive fusion protein attachment receptor (SNARE) molecules are involved in the homotypic fusion of ISGs. Interestingly, the SNARE molecules mediating the exocytosis of MSGs in neuroendocrine cells, syntaxin 1, SNAP-25, and VAMP2, were not involved in homotypic ISG fusion. Instead, we have identified syntaxin 6 as a component of the core machinery responsible for homotypic ISG fusion. Subcellular fractionation studies and indirect immunofluorescence microscopy show that syntaxin 6 is sorted away during the maturation of ISGs to MSGs. Although, syntaxin 6 on ISG membranes is associated with SNAP-25 and SNAP-29/GS32, we could not find evidence that these target (t)-SNARE molecules are involved in homotypic ISG fusion. Nor could we find any involvement for the vesicle (v)-SNARE VAMP4, which is known to be associated with syntaxin 6. Importantly, we have shown that homotypic fusion requires the function of syntaxin 6 on both donor as well as acceptor membranes, which suggests that t-t-SNARE interactions, either direct or indirect, may be required during fusion of ISG membranes.
Figures


Similar articles
-
A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function.EMBO J. 2000 Dec 1;19(23):6453-64. doi: 10.1093/emboj/19.23.6453. EMBO J. 2000. PMID: 11101518 Free PMC article.
-
Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting.Biochem J. 2001 Aug 1;357(Pt 3):625-34. doi: 10.1042/0264-6021:3570625. Biochem J. 2001. PMID: 11463334 Free PMC article.
-
Localization and function of soluble N-ethylmaleimide-sensitive factor attachment protein-25 and vesicle-associated membrane protein-2 in functioning gastric parietal cells.J Biol Chem. 2002 Dec 20;277(51):50030-5. doi: 10.1074/jbc.M207694200. Epub 2002 Oct 16. J Biol Chem. 2002. PMID: 12386166
-
Syntaxin 6: the promiscuous behaviour of a SNARE protein.Traffic. 2001 Sep;2(9):606-11. doi: 10.1034/j.1600-0854.2001.20903.x. Traffic. 2001. PMID: 11555414 Review.
-
Functional regulation of syntaxin-1: An underlying mechanism mediating exocytosis in neuroendocrine cells.Front Endocrinol (Lausanne). 2023 Jan 19;14:1096365. doi: 10.3389/fendo.2023.1096365. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36742381 Free PMC article. Review.
Cited by
-
Kalirin/Trio Rho guanine nucleotide exchange factors regulate a novel step in secretory granule maturation.Mol Biol Cell. 2007 Dec;18(12):4813-25. doi: 10.1091/mbc.e07-05-0503. Epub 2007 Sep 19. Mol Biol Cell. 2007. PMID: 17881726 Free PMC article.
-
GGA function is required for maturation of neuroendocrine secretory granules.EMBO J. 2006 Apr 19;25(8):1590-602. doi: 10.1038/sj.emboj.7601067. Epub 2006 Apr 6. EMBO J. 2006. PMID: 16601685 Free PMC article.
-
Calnuc plays a role in dynamic distribution of Galphai but not Gbeta subunits and modulates ACTH secretion in AtT-20 neuroendocrine secretory cells.Mol Neurodegener. 2009 Mar 25;4:15. doi: 10.1186/1750-1326-4-15. Mol Neurodegener. 2009. PMID: 19320978 Free PMC article.
-
An efficient two-step subcellular fractionation method for the enrichment of insulin granules from INS-1 cells.Biophys Rep. 2015;1:34-40. doi: 10.1007/s41048-015-0008-x. Epub 2015 Aug 7. Biophys Rep. 2015. PMID: 26942217 Free PMC article.
-
The dense-core vesicle maturation protein CCCP-1 binds RAB-2 and membranes through its C-terminal domain.Traffic. 2017 Nov;18(11):720-732. doi: 10.1111/tra.12507. Epub 2017 Sep 13. Traffic. 2017. PMID: 28755404 Free PMC article.
References
-
- Advani RJ, Bae H-R, Bock JB, Chao DS, Doung Y-C, Prekeris R, Yoo J-S, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
-
- Austin C, Hinners I, Tooze SA. Direct and GTP-dependent Interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J Biol Chem. 2000;275:21862–21869. - PubMed
-
- Banerjee A, Barry VA, Bibhuti DR, Martin TFJ. N-Ethylmaleimide-sensitive FACTOR acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. - PubMed
-
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesices at presynaptic active zones. Science. 1992;257:255–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

