Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis
- PMID: 11390621
- PMCID: PMC114335
- DOI: 10.1128/JVI.75.13.6193-6198.2001
Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis
Abstract
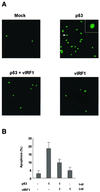
Kaposi's sarcoma-associated herpesvirus (KSHV) is related to the development of Kaposi's sarcoma. Open reading frame K9 of KSHV encodes viral interferon regulatory factor 1 (vIRF1), which functions as a repressor of interferon- and IRF1-mediated signal transduction. In addition, vIRF1 acts as an oncogene to induce cellular transformation. Here we show that vIRF1 directly associates with the tumor suppressor p53 and represses its functions. The vIRF1 interaction domains of p53 are the DNA binding domain (amino acids [aa] 100 to 300) and the tetramerization domain (aa 300 to 393). p53 interacts with the central region (aa 152 to 360) of vIRF1. vIRF1 suppresses p53-dependent transcription and deregulates its apoptotic activity. These results suggest that vIRF1 may regulate cellular function by inhibiting p53.
Figures


Similar articles
-
Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death.J Virol. 2002 Sep;76(17):8797-807. doi: 10.1128/jvi.76.17.8797-8807.2002. J Virol. 2002. PMID: 12163600 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 1 inhibits transforming growth factor-beta signaling.Cancer Res. 2005 Mar 1;65(5):1738-47. doi: 10.1158/0008-5472.CAN-04-2374. Cancer Res. 2005. PMID: 15753369
-
Inhibition of the ATM/p53 signal transduction pathway by Kaposi's sarcoma-associated herpesvirus interferon regulatory factor 1.J Virol. 2006 Mar;80(5):2257-66. doi: 10.1128/JVI.80.5.2257-2266.2006. J Virol. 2006. PMID: 16474133 Free PMC article.
-
Identification of the DNA sequence interacting with Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 1.J Virol. 2007 Nov;81(22):12680-4. doi: 10.1128/JVI.00556-07. Epub 2007 Sep 12. J Virol. 2007. PMID: 17855527 Free PMC article.
-
Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer.J Virol. 2013 Sep;87(17):9398-410. doi: 10.1128/JVI.03315-12. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785197 Free PMC article. Review.
Cited by
-
Viral interferon regulatory factors.J Interferon Cytokine Res. 2009 Sep;29(9):621-7. doi: 10.1089/jir.2009.0067. J Interferon Cytokine Res. 2009. PMID: 19715458 Free PMC article. Review.
-
Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases.Microorganisms. 2020 Mar 11;8(3):388. doi: 10.3390/microorganisms8030388. Microorganisms. 2020. PMID: 32168836 Free PMC article. Review.
-
Human herpesvirus 6 open reading frame U14 protein and cellular p53 interact with each other and are contained in the virion.J Virol. 2005 Oct;79(20):13037-46. doi: 10.1128/JVI.79.20.13037-13046.2005. J Virol. 2005. PMID: 16189006 Free PMC article.
-
Murine herpesvirus-68-related growth factors treatment correlates with decrease of p53 and HIF-1α protein levels.Pathog Dis. 2023 Jan 17;81:ftad004. doi: 10.1093/femspd/ftad004. Pathog Dis. 2023. PMID: 36997335 Free PMC article.
-
Modulation of Immune System by Kaposi's Sarcoma-Associated Herpesvirus: Lessons from Viral Evasion Strategies.Front Microbiol. 2012 Mar 5;3:44. doi: 10.3389/fmicb.2012.00044. eCollection 2012. Front Microbiol. 2012. PMID: 22403573 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Friborg J, Kong W, Hottiger M O, Nabel G J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. - PubMed
-
- Gao S, Boshoff J C, Jayachandra S, Weiss R A, Chang Y, Moore P S. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15:1979–1985. - PubMed
-
- Gorczyca W, Bigman K, Mittelman A, Ahmed T, Gong J, Melamed M R, Darzynkiewicz Z. Induction of DNA strand breaks associated with apoptosis during treatment of leukemias. Leukemia. 1993;7:659–670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

