Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions
- PMID: 11390577
- PMCID: PMC114291
- DOI: 10.1128/JVI.75.13.5752-5761.2001
Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions
Abstract
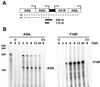
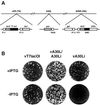
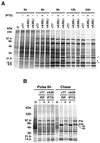
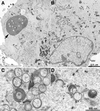
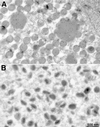
The previously uncharacterized A30L gene of vaccinia virus has orthologs in all vertebrate poxviruses but no recognizable nonpoxvirus homologs or functional motifs. We determined that the A30L gene was regulated by a late promoter and encoded a protein of approximately 9 kDa. Immunoelectron microscopy of infected cells indicated that the A30L protein was associated with viroplasm enclosed by crescent and immature virion membranes. The A30L protein was also present in mature virions and was partially released by treatment with a nonionic detergent and reducing agent, consistent with a location in the matrix between the core and envelope. To determine the role of the A30L protein, we constructed a stringent conditional lethal mutant with an inducible A30L gene. In the absence of inducer, synthesis of viral early and late proteins occurred but the proteolytic processing of certain core proteins was inhibited, suggesting an assembly block. Inhibition of virus maturation was confirmed by electron microscopy. Under nonpermissive conditions, we observed aberrant large, dense, granular masses of viroplasm with clearly defined margins; viral crescent membranes that appeared normal except for their location at a distance from viroplasm; empty immature virions; and an absence of mature virions. The data indicated that the A30L protein is needed for vaccinia virus morphogenesis, specifically the association of the dense viroplasm with viral membranes.
Figures


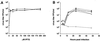

Similar articles
-
Vaccinia virus G7L protein Interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2003 Mar;77(6):3418-29. doi: 10.1128/jvi.77.6.3418-3429.2003. J Virol. 2003. PMID: 12610117 Free PMC article.
-
A complex of seven vaccinia virus proteins conserved in all chordopoxviruses is required for the association of membranes and viroplasm to form immature virions.Virology. 2004 Dec 20;330(2):447-59. doi: 10.1016/j.virol.2004.10.008. Virology. 2004. PMID: 15567438
-
Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis.Virology. 2000 Dec 5;278(1):244-52. doi: 10.1006/viro.2000.0656. Virology. 2000. PMID: 11112499
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
-
Vaccinia virus proteolysis--a review.Rev Med Virol. 2006 May-Jun;16(3):187-202. doi: 10.1002/rmv.499. Rev Med Virol. 2006. PMID: 16710840 Free PMC article. Review.
Cited by
-
Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles.J Virol. 2003 Aug;77(16):9052-68. doi: 10.1128/jvi.77.16.9052-9068.2003. J Virol. 2003. PMID: 12885921 Free PMC article.
-
Structure/Function analysis of the vaccinia virus F18 phosphoprotein, an abundant core component required for virion maturation and infectivity.J Virol. 2010 Jul;84(13):6846-60. doi: 10.1128/JVI.00399-10. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392848 Free PMC article.
-
Characterization of the myxoma virus M118L protein: a novel essential poxvirus IMV-associated protein.Virus Genes. 2001 Dec;23(3):303-13. doi: 10.1023/a:1012573306916. Virus Genes. 2001. PMID: 11778698
-
Vaccinia virus G7L protein Interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2003 Mar;77(6):3418-29. doi: 10.1128/jvi.77.6.3418-3429.2003. J Virol. 2003. PMID: 12610117 Free PMC article.
-
Modulation of Early Host Innate Immune Response by an Avipox Vaccine Virus' Lateral Body Protein.Biomedicines. 2020 Dec 19;8(12):634. doi: 10.3390/biomedicines8120634. Biomedicines. 2020. PMID: 33352813 Free PMC article.
References
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Boulter E A, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

