The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism
- PMID: 11375938
- PMCID: PMC1083888
- DOI: 10.1093/embo-reports/kve094
The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism
Abstract
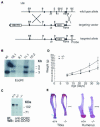
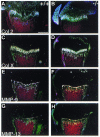
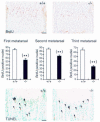
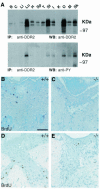
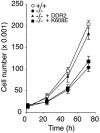
The discoidin domain receptor 2 (DDR2) is a member of a subfamily of receptor tyrosine kinases whose ligands are fibrillar collagens, and is widely expressed in postnatal tissues. We have generated DDR2-deficient mice to establish the in vivo functions of this receptor, which have remained obscure. These mice exhibit dwarfism and shortening of long bones. This phenotype appears to be caused by reduced chondrocyte proliferation, rather than aberrant differentiation or function. In a skin wound healing model, DDR2-/- mice exhibit a reduced proliferative response compared with wild-type littermates. In vitro, fibroblasts derived from DDR2-/- mutants proliferate more slowly than wild-type fibroblasts, a defect that is rescued by introduction of wild-type but not kinase-dead DDR2 receptor. Together our results suggest that DDR2 acts as an extracellular matrix sensor to modulate cell proliferation.
Figures

Similar articles
-
Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2.J Biol Chem. 2002 Feb 1;277(5):3606-13. doi: 10.1074/jbc.M107571200. Epub 2001 Nov 26. J Biol Chem. 2002. PMID: 11723120
-
Discoidin domain receptor 2 (DDR2) regulates proliferation of endochondral cells in mice.Biochem Biophys Res Commun. 2012 Oct 26;427(3):611-7. doi: 10.1016/j.bbrc.2012.09.106. Epub 2012 Sep 26. Biochem Biophys Res Commun. 2012. PMID: 23022180
-
An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation.J Bone Miner Res. 2011 Mar;26(3):604-17. doi: 10.1002/jbmr.225. J Bone Miner Res. 2011. PMID: 20734453
-
Role of discoidin domain receptor 2 in wound healing.Histol Histopathol. 2014 Nov;29(11):1355-64. doi: 10.14670/HH-29.1355. Epub 2014 Apr 29. Histol Histopathol. 2014. PMID: 24781958 Review.
-
Exploring the Cellular and Molecular Mechanism of Discoidin Domain Receptors (DDR1 and DDR2) in Bone Formation, Regeneration, and Its Associated Disease Conditions.Int J Mol Sci. 2023 Oct 4;24(19):14895. doi: 10.3390/ijms241914895. Int J Mol Sci. 2023. PMID: 37834343 Free PMC article. Review.
Cited by
-
Discoidin Receptor 2 Controls Bone Formation and Marrow Adipogenesis.J Bone Miner Res. 2016 Dec;31(12):2193-2203. doi: 10.1002/jbmr.2893. Epub 2016 Oct 31. J Bone Miner Res. 2016. PMID: 27341689 Free PMC article.
-
Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling.Structure. 2012 Apr 4;20(4):688-97. doi: 10.1016/j.str.2012.02.011. Epub 2012 Apr 3. Structure. 2012. PMID: 22483115 Free PMC article.
-
Transcriptome analysis reveals an unexpected role of a collagen tyrosine kinase receptor gene, Ddr2, as a regulator of ovarian function.Physiol Genomics. 2009 Oct 7;39(2):120-9. doi: 10.1152/physiolgenomics.00073.2009. Epub 2009 Aug 11. Physiol Genomics. 2009. PMID: 19671659 Free PMC article.
-
DDR2, a discoidin domain receptor, is a marker of periosteal osteoblast and osteoblast progenitors.J Bone Miner Metab. 2020 Sep;38(5):670-677. doi: 10.1007/s00774-020-01108-y. Epub 2020 May 15. J Bone Miner Metab. 2020. PMID: 32415375 Free PMC article.
-
Discoidin domain receptor functions in physiological and pathological conditions.Int Rev Cell Mol Biol. 2014;310:39-87. doi: 10.1016/B978-0-12-800180-6.00002-5. Int Rev Cell Mol Biol. 2014. PMID: 24725424 Free PMC article. Review.
References
-
- Alves F., Vogel, W., Mossie, K., Millauer, B., Hofler H. and Ullrich, A. (1995) Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene, 10, 609–618. - PubMed
-
- Ankoma-Sey V., Matli, M., Chang, K.B., Lalazar, A., Donner, D.B., Wong, L., Warren, R.S. and Friedman, S.L. (1998) Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene, 17, 115–121. - PubMed
-
- Barker K.T., Martindale, J.E., Mitchell, P.J., Kamalati, T., Page, M.J., Phippard, D.J., Dale, T.C., Gusterson, B.A. and Crompton, M.R. (1995) Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumours. Oncogene, 10, 569–575. - PubMed
-
- Brückner K., Labrador, J.P., Scheiffele, P., Herb, A., Seeburg, P.H. and Klein, R. (1999) EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron, 22, 511–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

