Developmental plasticity of CNS microglia
- PMID: 11371643
- PMCID: PMC33462
- DOI: 10.1073/pnas.111152498
Developmental plasticity of CNS microglia
Abstract
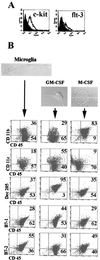
Microglia arise from CD45(+) bone marrow precursors that colonize the fetal brain and play a key role in central nervous system inflammatory conditions. We report that parenchymal microglia are uncommitted myeloid progenitors of immature dendritic cells and macrophages by several criteria, including surface expression of "empty" class II MHC protein and their cysteine protease (cathepsin) profile. Microglia express receptors for stem cell factor and can be skewed toward more dendritic cell or macrophage-like profiles in response to the lineage growth factors granulocyte/macrophage colony-stimulating factor or macrophage colony-stimulating factor. Thus, in contrast to other organs, where terminally differentiated populations of resident dendritic cells and/or macrophages outnumber colonizing precursors, the majority of microglia within the brain remain in an undifferentiated state.
Figures

Similar articles
-
In vitro induction of inhibitory macrophage differentiation by granulocyte-macrophage colony-stimulating factor, stem cell factor and interferon-gamma from lineage phenotypes-negative c-kit-positive murine hematopoietic progenitor cells.Immunol Lett. 2004 Feb 15;91(2-3):221-7. doi: 10.1016/j.imlet.2003.12.008. Immunol Lett. 2004. PMID: 15019293
-
Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow.J Immunol. 1992 Oct 15;149(8):2681-8. J Immunol. 1992. PMID: 1383322
-
Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state.J Immunol Methods. 2005 May;300(1-2):32-46. doi: 10.1016/j.jim.2005.02.011. Epub 2005 Apr 26. J Immunol Methods. 2005. PMID: 15893321
-
Embryonic hematopoiesis.Blood Cells Mol Dis. 2013 Dec;51(4):226-31. doi: 10.1016/j.bcmd.2013.08.004. Epub 2013 Sep 13. Blood Cells Mol Dis. 2013. PMID: 24041595 Review.
-
Ontogeny and functions of central nervous system macrophages.J Immunol. 2014 Sep 15;193(6):2615-21. doi: 10.4049/jimmunol.1400716. J Immunol. 2014. PMID: 25193935 Free PMC article. Review.
Cited by
-
Role of the immunogenic and tolerogenic subsets of dendritic cells in multiple sclerosis.Mediators Inflamm. 2015;2015:513295. doi: 10.1155/2015/513295. Epub 2015 Jan 29. Mediators Inflamm. 2015. PMID: 25705093 Free PMC article. Review.
-
Gene therapy and targeted toxins for glioma.Curr Gene Ther. 2005 Dec;5(6):535-57. doi: 10.2174/156652305774964631. Curr Gene Ther. 2005. PMID: 16457645 Free PMC article. Review.
-
TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis.PLoS Med. 2007 Apr;4(4):e124. doi: 10.1371/journal.pmed.0040124. PLoS Med. 2007. PMID: 17425404 Free PMC article.
-
Is Alzheimer disease a failure of mobilizing immune defense? Lessons from cognitively fit oldest-old.Dialogues Clin Neurosci. 2019 Mar;21(1):7-19. doi: 10.31887/DCNS.2019.21.1/vharoutunian. Dialogues Clin Neurosci. 2019. PMID: 31607776 Free PMC article.
-
Inflammation in the central nervous system: the role for dendritic cells.Brain Pathol. 2003 Jan;13(1):23-33. doi: 10.1111/j.1750-3639.2003.tb00003.x. Brain Pathol. 2003. PMID: 12580542 Free PMC article. Review.
References
-
- Lawson L J, Perry V H, Dri P, Gordon S. Neuroscience. 1990;39:151–170. - PubMed
-
- Aloisi F. Adv Exp Med Biol. 1999;468:123–133. - PubMed
-
- Hickey W F, Kimura H. Science. 1988;239:290–292. - PubMed
-
- Wegiel J, Wisniewski H M, Dziewiatkowski J, Tarnawski M, Kozielski R, Trenkner E, Wiktor-Jedrzejczak W. Brain Res. 1998;804:135–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

