An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense
- PMID: 11353867
- PMCID: PMC33500
- DOI: 10.1073/pnas.111440998
An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense
Abstract
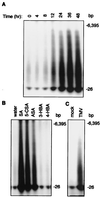
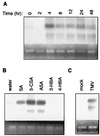
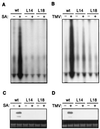
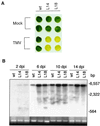
Plants contain RNA-dependent RNA polymerase (RdRP) activities that synthesize short cRNAs by using cellular or viral RNAs as templates. During studies of salicylic acid (SA)-induced resistance to viral pathogens, we recently found that the activity of a tobacco RdRP was increased in virus-infected or SA-treated plants. Biologically active SA analogs capable of activating plant defense response also induced the RdRP activity, whereas biologically inactive analogs did not. A tobacco RdRP gene, NtRDRP1, was isolated and found to be induced both by virus infection and by treatment with SA or its biologically active analogs. Tobacco lines deficient in the inducible RDRP activity were obtained by expressing antisense RNA for the NtRDRP1 gene in transgenic plants. When infected by tobacco mosaic virus, these transgenic plants accumulated significantly higher levels of viral RNA and developed more severe disease symptoms than wild-type plants. After infection by a strain of potato virus X that does not spread in wild-type tobacco plants, the transgenic NtRDRP1 antisense plants accumulated virus and developed symptoms not only locally in inoculated leaves but also systemically in upper uninoculated leaves. These results strongly suggest that inducible RdRP activity plays an important role in plant antiviral defense.
Figures


Similar articles
-
Analysis of the involvement of an inducible Arabidopsis RNA-dependent RNA polymerase in antiviral defense.Mol Plant Microbe Interact. 2003 Mar;16(3):206-16. doi: 10.1094/MPMI.2003.16.3.206. Mol Plant Microbe Interact. 2003. PMID: 12650452
-
Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana.BMC Plant Biol. 2016 Jan 13;16:15. doi: 10.1186/s12870-016-0705-8. BMC Plant Biol. 2016. PMID: 26757721 Free PMC article.
-
RNA silencing-related genes contribute to tolerance of infection with potato virus X and Y in a susceptible tomato plant.Virol J. 2020 Oct 8;17(1):149. doi: 10.1186/s12985-020-01414-x. Virol J. 2020. PMID: 33032637 Free PMC article.
-
Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco.Plant J. 2006 Oct;48(2):217-27. doi: 10.1111/j.1365-313X.2006.02861.x. Plant J. 2006. PMID: 17018032
-
RNA-dependent RNA polymerase 1 in potato (Solanum tuberosum) and its relationship to other plant RNA-dependent RNA polymerases.Sci Rep. 2016 Mar 16;6:23082. doi: 10.1038/srep23082. Sci Rep. 2016. PMID: 26979928 Free PMC article.
Cited by
-
Viral and cellular factors involved in Phloem transport of plant viruses.Front Plant Sci. 2013 May 24;4:154. doi: 10.3389/fpls.2013.00154. eCollection 2013. Front Plant Sci. 2013. PMID: 23745125 Free PMC article.
-
Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection.Plant Cell. 2010 Feb;22(2):481-96. doi: 10.1105/tpc.109.073056. Epub 2010 Feb 26. Plant Cell. 2010. PMID: 20190077 Free PMC article.
-
RNase If -treated quantitative PCR for dsRNA quantitation of RNAi trait in genetically modified crops.BMC Biotechnol. 2018 Jan 17;18(1):3. doi: 10.1186/s12896-018-0413-6. BMC Biotechnol. 2018. PMID: 29343265 Free PMC article.
-
Structural and genetic requirements for the biogenesis of tobacco rattle virus-derived small interfering RNAs.J Virol. 2008 Jun;82(11):5167-77. doi: 10.1128/JVI.00272-08. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353962 Free PMC article.
-
TMV-Cg Coat Protein stabilizes DELLA proteins and in turn negatively modulates salicylic acid-mediated defense pathway during Arabidopsis thaliana viral infection.BMC Plant Biol. 2014 Aug 3;14:210. doi: 10.1186/s12870-014-0210-x. BMC Plant Biol. 2014. PMID: 25084837 Free PMC article.
References
-
- Astier-Manifacier S, Cornuet P. C R Hebd Seances Acad Sci D. 1978;287:1043–1046. - PubMed
-
- Astier-Manifacier S, Cornuet P. Biochim Biophys Acta. 1971;232:484–493. - PubMed
-
- Boege F. Biosci Rep. 1982;2:379–389. - PubMed
-
- Boege F, Rohde W, Sanger H L. Biosci Rep. 1982;2:185–194. - PubMed
-
- Dorssers L, Zabel P, van der Meer J, van Kammen A. Virology. 1982;116:236–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

