Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation
- PMID: 11342593
- PMCID: PMC2193434
- DOI: 10.1084/jem.193.9.1087
Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation
Abstract
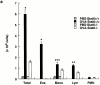
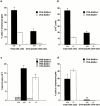
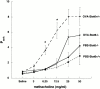
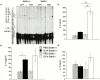
Antigen-specific CD4 T helper type 2 (Th2) cells play a pivotal role in the induction of allergic asthma, but the mechanisms regulating their recruitment into the airways are unknown. Signal transducer and activator of transcription factor (Stat)6 is a transcription factor essential for Th2 cell differentiation. Here we show that Stat6 also controls Th2 cell recruitment and effector function in allergic inflammation in vivo. To isolate the role of Stat6 in regulating Th2 cell trafficking and effector function from its role in Th2 cell differentiation, we used a murine model of asthma in which in vitro-differentiated Stat6(+/+) antigen-specific Th2 cells were adoptively transferred into naive Stat6(-/-) and Stat6(+/+) mice followed by aerosol antigen challenge. We found that all of the features of asthma, including Th2 cell accumulation, Th2 and eosinophil-active chemokine production, and airway eosinophilia, mucus production, and hyperresponsiveness seen in Stat6(+/+) mice, were dramatically absent in Stat6(-/)- mice that received Stat6(+/)+ antigen-specific Th2 cells. Our findings establish Stat6 as essential for Th2 cell trafficking and effector function and suggest that interruption of Stat6 signaling in resident cells of the lung is a novel approach to asthma therapy.
Figures

Similar articles
-
STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation.Int Immunol. 2004 Oct;16(10):1497-505. doi: 10.1093/intimm/dxh151. Epub 2004 Sep 6. Int Immunol. 2004. PMID: 15351784
-
Transfer of in vivo primed transgenic T cells supports allergic lung inflammation and FIZZ1 and Ym1 production in an IL-4Rα and STAT6 dependent manner.BMC Immunol. 2011 Oct 20;12:60. doi: 10.1186/1471-2172-12-60. BMC Immunol. 2011. PMID: 22014099 Free PMC article.
-
Dissociation of T helper type 2 cytokine-dependent airway lesions from signal transducer and activator of transcription 6 signalling in experimental chronic asthma.Clin Exp Allergy. 2003 May;33(5):688-95. doi: 10.1046/j.1365-2222.2003.01647.x. Clin Exp Allergy. 2003. PMID: 12752600
-
The failure of STAT6-deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5.Am J Respir Crit Care Med. 1999 Oct;160(4):1283-91. doi: 10.1164/ajrccm.160.4.9809065. Am J Respir Crit Care Med. 1999. PMID: 10508820
-
STAT4 and STAT6, their role in cellular and humoral immunity and in diverse human diseases.Int Rev Immunol. 2024;43(6):394-418. doi: 10.1080/08830185.2024.2395274. Epub 2024 Aug 26. Int Rev Immunol. 2024. PMID: 39188021 Review.
Cited by
-
Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency.Nat Immunol. 2002 Apr;3(4):347-53. doi: 10.1038/ni773. Epub 2002 Mar 11. Nat Immunol. 2002. PMID: 11887181 Free PMC article.
-
G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action.Pharmacol Rev. 2020 Jan;72(1):1-49. doi: 10.1124/pr.118.016899. Pharmacol Rev. 2020. PMID: 31767622 Free PMC article. Review.
-
Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper-responsiveness?Front Physiol. 2012 Jul 31;3:312. doi: 10.3389/fphys.2012.00312. eCollection 2012. Front Physiol. 2012. PMID: 23060800 Free PMC article.
-
Absence of the adaptor protein Shb potentiates the T helper type 2 response in a mouse model of atopic dermatitis.Immunology. 2014 Sep;143(1):33-41. doi: 10.1111/imm.12286. Immunology. 2014. PMID: 24645804 Free PMC article.
-
Immunoregulatory roles of eosinophils: a new look at a familiar cell.Clin Exp Allergy. 2008 Aug;38(8):1254-63. doi: 10.1111/j.1365-2222.2008.03037.x. Clin Exp Allergy. 2008. PMID: 18727793 Free PMC article. Review.
References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
-
- Del Prete G.F., De Carli M., D'Elios M.M., Maestrelli P., Ricci M., Fabbri L., Romagnani S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur. J. Immunol. 1993;23:1445–1449. - PubMed
-
- Robinson D.S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A.M., Corrigan C., Durham S.R., Kay A.B. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Eng. J. Med. 1992;326:298–304. - PubMed
-
- Walker C., Bode E., Boer L., Hansel T., Blaser K., Virchow C., Jr. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am. Rev. Respir. Dis. 1992;146:109–115. - PubMed
-
- Bruselle G.J., Kips G., Bluethmann G., Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wildtype and IL-4 deficient mice. Am. J. Respir. Cell Biol. 1995;12:254–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

