c-Myc is a critical target for c/EBPalpha in granulopoiesis
- PMID: 11340171
- PMCID: PMC87031
- DOI: 10.1128/MCB.21.11.3789-3806.2001
c-Myc is a critical target for c/EBPalpha in granulopoiesis
Abstract
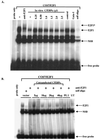
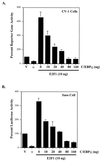
CCAAT/enhancer binding protein alpha (C/EBPalpha) is an integral factor in the granulocytic developmental pathway, as myeloblasts from C/EBPalpha-null mice exhibit an early block in differentiation. Since mice deficient for known C/EBPalpha target genes do not exhibit the same block in granulocyte maturation, we sought to identify additional C/EBPalpha target genes essential for myeloid cell development. To identify such genes, we used both representational difference analysis and oligonucleotide array analysis with RNA derived from a C/EBPalpha-inducible myeloid cell line. From each of these independent screens, we identified c-Myc as a C/EBPalpha negatively regulated gene. We mapped an E2F binding site in the c-Myc promoter as the cis-acting element critical for C/EBPalpha negative regulation. The identification of c-Myc as a C/EBPalpha target gene is intriguing, as it has been previously shown that down-regulation of c-Myc can induce myeloid differentiation. Here we show that stable expression of c-Myc from an exogenous promoter not responsive to C/EBPalpha-mediated down-regulation forces myeloblasts to remain in an undifferentiated state. Therefore, C/EBPalpha negative regulation of c-Myc is critical for allowing early myeloid precursors to enter a differentiation pathway. This is the first report to demonstrate that C/EBPalpha directly affects the level of c-Myc expression and, thus, the decision of myeloid blasts to enter into the granulocytic differentiation pathway.
Figures




Similar articles
-
A direct role of transcription factor E2F in c-myc gene expression during granulocytic and macrophage-like differentiation of HL60 cells.Cell Growth Differ. 1995 Mar;6(3):229-37. Cell Growth Differ. 1995. PMID: 7794791
-
Dual control of myc expression through a single DNA binding site targeted by ets family proteins and E2F-1.Oncogene. 1994 Feb;9(2):405-15. Oncogene. 1994. PMID: 8290253
-
Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway.J Biol Chem. 2005 Apr 1;280(13):12621-9. doi: 10.1074/jbc.M408442200. Epub 2005 Jan 21. J Biol Chem. 2005. PMID: 15664994
-
Regulation of granulocyte and monocyte differentiation by CCAAT/enhancer binding protein alpha.Blood Cells Mol Dis. 2003 Nov-Dec;31(3):338-41. doi: 10.1016/s1079-9796(03)00135-9. Blood Cells Mol Dis. 2003. PMID: 14636649 Review.
-
LEF-1 is a decisive transcription factor in neutrophil granulopoiesis.Ann N Y Acad Sci. 2007 Jun;1106:143-51. doi: 10.1196/annals.1392.012. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360796 Review.
Cited by
-
C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence.Nat Cell Biol. 2013 Apr;15(4):385-94. doi: 10.1038/ncb2698. Epub 2013 Mar 17. Nat Cell Biol. 2013. PMID: 23502316 Free PMC article.
-
Strategies to generate functionally normal neutrophils to reduce infection and infection-related mortality in cancer chemotherapy.Pharmacol Ther. 2019 Dec;204:107403. doi: 10.1016/j.pharmthera.2019.107403. Epub 2019 Aug 27. Pharmacol Ther. 2019. PMID: 31470030 Free PMC article. Review.
-
Molecular mechanisms underlying deregulation of C/EBPalpha in acute myeloid leukemia.Int J Hematol. 2010 May;91(4):557-68. doi: 10.1007/s12185-010-0573-1. Epub 2010 Apr 27. Int J Hematol. 2010. PMID: 20422469 Review.
-
GATA-1-mediated proliferation arrest during erythroid maturation.Mol Cell Biol. 2003 Jul;23(14):5031-42. doi: 10.1128/MCB.23.14.5031-5042.2003. Mol Cell Biol. 2003. PMID: 12832487 Free PMC article.
-
Granulopoiesis requires increased C/EBPα compared to monopoiesis, correlated with elevated Cebpa in immature G-CSF receptor versus M-CSF receptor expressing cells.PLoS One. 2014 Apr 21;9(4):e95784. doi: 10.1371/journal.pone.0095784. eCollection 2014. PLoS One. 2014. PMID: 24752325 Free PMC article.
References
-
- Antonson P, Pray M G, Jacobsson A, Xanthopoulos K G. Myc inhibits CCAAT/enhancer-binding protein alpha-gene expression in HIB-1B hibernoma cells through interactions with the core promoter region. Eur J Biochem. 1995;232:397–403. - PubMed
-
- Battey J, Moulding C, Taub R, Murphy W, Stewart T, Potter H, Lenoir G, Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983;34:779–787. - PubMed
-
- Behre G, Smith L T, Tenen D G. Use of a promoterless Renilla luciferase vector as an internal control plasmid for transient co-transfection assays of Ras-mediated transcription activation. BioTechniques. 1999;26:24–28. - PubMed
-
- Bellon T, Perrotti D, Calabretta B. Granulocytic differentiation of normal hematopoietic precursor cells induced by transcription factor PU.1 correlates with negative regulation of the c-myb promoter. Blood. 1997;90:1828–1839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
