Aggresomes resemble sites specialized for virus assembly
- PMID: 11331297
- PMCID: PMC2190574
- DOI: 10.1083/jcb.153.3.449
Aggresomes resemble sites specialized for virus assembly
Abstract
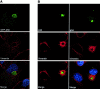
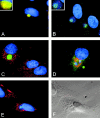
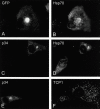
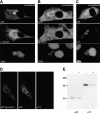
The large cytoplasmic DNA viruses such as poxviruses, iridoviruses, and African swine fever virus (ASFV) assemble in discrete perinuclear foci called viral factories. Factories exclude host proteins, suggesting that they are novel subcellular structures induced by viruses. Novel perinuclear structures, called aggresomes are also formed by cells in response to misfolded protein (Johnston, J.A., C.L. Ward, and R.R. Kopito. 1998. J. Cell Biol. 143:1883--1898; García-Mata, R., Z. Bebök, E.J. Sorscher, and E.S. Sztul. 1999. J. Cell Biol. 146:1239--1254). In this study, we have investigated whether aggresomes and viral factories are related structures. Aggresomes were compared with viral factories produced by ASFV. Aggresomes and viral factories were located close to the microtubule organizing center and required an intact microtubular network for assembly. Both structures caused rearrangement of intermediate filaments and the collapse of vimentin into characteristic cages, and both recruited mitochondria and cellular chaperones. Given that ASFV factories resemble aggresomes, it is possible that a cellular response originally designed to reduce the toxicity of misfolded proteins is exploited by cytoplasmic DNA viruses to concentrate structural proteins at virus assembly sites.
Figures

Similar articles
-
Formation of aggresome-like structures in herpes simplex virus type 2-infected cells and a potential role in virus assembly.Exp Cell Res. 2004 Oct 1;299(2):486-97. doi: 10.1016/j.yexcr.2004.06.010. Exp Cell Res. 2004. PMID: 15350546
-
Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II.J Virol. 2005 Sep;79(18):11766-75. doi: 10.1128/JVI.79.18.11766-11775.2005. J Virol. 2005. PMID: 16140754 Free PMC article.
-
Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera.J Cell Biol. 1999 Sep 20;146(6):1239-54. doi: 10.1083/jcb.146.6.1239. J Cell Biol. 1999. PMID: 10491388 Free PMC article.
-
Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design?Annu Rev Microbiol. 2007;61:149-67. doi: 10.1146/annurev.micro.57.030502.090836. Annu Rev Microbiol. 2007. PMID: 17896875 Review.
-
African swine fever virus organelle rearrangements.Virus Res. 2013 Apr;173(1):76-86. doi: 10.1016/j.virusres.2012.12.014. Epub 2013 Jan 3. Virus Res. 2013. PMID: 23291273 Review.
Cited by
-
Analysis of HDAC6 and BAG3-aggresome pathways in African swine fever viral factory formation.Viruses. 2015 Apr 8;7(4):1823-31. doi: 10.3390/v7041823. Viruses. 2015. PMID: 25856634 Free PMC article.
-
The subcellular distribution of multigene family 110 proteins of African swine fever virus is determined by differences in C-terminal KDEL endoplasmic reticulum retention motifs.J Virol. 2004 Apr;78(7):3710-21. doi: 10.1128/jvi.78.7.3710-3721.2004. J Virol. 2004. PMID: 15016891 Free PMC article.
-
Membrane association facilitates the correct processing of pp220 during production of the major matrix proteins of African swine fever virus.J Virol. 2003 Feb;77(3):1682-90. doi: 10.1128/jvi.77.3.1682-1690.2003. J Virol. 2003. PMID: 12525602 Free PMC article.
-
Research progress on the proteins involved in African swine fever virus infection and replication.Front Immunol. 2022 Jul 22;13:947180. doi: 10.3389/fimmu.2022.947180. eCollection 2022. Front Immunol. 2022. PMID: 35935977 Free PMC article. Review.
-
DNA virus replication compartments.J Virol. 2014 Feb;88(3):1404-20. doi: 10.1128/JVI.02046-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257611 Free PMC article. Review.
References
-
- Chen M., Goorha R., Murti K.G. Interaction of frog virus-3 with the cytomatrix. IV. Phosphorylation of vimentin precedes the reorganisation of intermediate filaments around the virus assembly sites. J. Gen. Virol. 1986;67:915–922. - PubMed
-
- Chou Y.H., Bischoff J.R., Beach D., Goldman R.D. Intermediate filament reorganisation during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–1071. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

