The P450-1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis
- PMID: 11320210
- PMCID: PMC33300
- DOI: 10.1073/pnas.091096298
The P450-1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis
Abstract
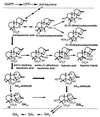
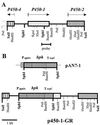
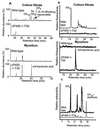
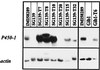
Recent studies have shown that the genes of the gibberellin (GA) biosynthesis pathway in the fungus Gibberella fujikuroi are organized in a cluster of at least seven genes. P450-1 is one of four cytochrome P450 monooxygenase genes in this cluster. Disruption of the P450-1 gene in the GA-producing wild-type strain IMI 58289 led to total loss of GA production. Analysis of the P450-1-disrupted mutants indicated that GA biosynthesis was blocked immediately after ent-kaurenoic acid. The function of the P450-1 gene product was investigated further by inserting the gene into mutants of G. fujikuroi that lack the entire GA gene cluster; the gene was highly expressed under GA production conditions in the absence of the other GA-biosynthesis genes. Cultures of transformants containing P450-1 converted ent-[(14)C]kaurenoic acid efficiently into [(14)C]GA(14), indicating that P450-1 catalyzes four sequential steps in the GA-biosynthetic pathway: 7beta-hydroxylation, contraction of ring B by oxidation at C-6, 3beta-hydroxylation, and oxidation at C-7. The GA precursors ent-7alpha-hydroxy[(14)C]kaurenoic acid, [(14)C]GA(12)-aldehyde, and [(14)C]GA(12) were also converted to [(14)C]GA(14). In addition, there is an indication that P450-1 may also be involved in the formation of the kaurenolides and fujenoic acids, which are by-products of GA biosynthesis in G. fujikuroi. Thus, P450-1 displays remarkable multifunctionality and may be responsible for the formation of 12 products.
Figures

Similar articles
-
Kaurenolides and fujenoic acids are side products of the gibberellin P450-1 monooxygenase in Gibberella fujikuroi.Phytochemistry. 2004 Apr;65(7):821-30. doi: 10.1016/j.phytochem.2004.02.007. Phytochemistry. 2004. PMID: 15081281
-
The P450-4 gene of Gibberella fujikuroi encodes ent-kaurene oxidase in the gibberellin biosynthesis pathway.Appl Environ Microbiol. 2001 Aug;67(8):3514-22. doi: 10.1128/AEM.67.8.3514-3522.2001. Appl Environ Microbiol. 2001. PMID: 11472927 Free PMC article.
-
Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi: des and P450-3 encode GA4 desaturase and the 13-hydroxylase, respectively.J Biol Chem. 2003 Aug 1;278(31):28635-43. doi: 10.1074/jbc.M301927200. Epub 2003 May 15. J Biol Chem. 2003. PMID: 12750377
-
Biochemical and molecular analyses of gibberellin biosynthesis in fungi.Biosci Biotechnol Biochem. 2006 Mar;70(3):583-90. doi: 10.1271/bbb.70.583. Biosci Biotechnol Biochem. 2006. PMID: 16556972 Review.
-
Biosynthesis of gibberellins in Gibberella fujikuroi: biomolecular aspects.Appl Microbiol Biotechnol. 1999 Sep;52(3):298-310. doi: 10.1007/s002530051524. Appl Microbiol Biotechnol. 1999. PMID: 10531641 Review.
Cited by
-
Cryptic and Stereospecific Hydroxylation, Oxidation, and Reduction in Platensimycin and Platencin Biosynthesis.J Am Chem Soc. 2019 Mar 6;141(9):4043-4050. doi: 10.1021/jacs.8b13452. Epub 2019 Feb 19. J Am Chem Soc. 2019. PMID: 30735041 Free PMC article.
-
Highlights in gibberellin research: A tale of the dwarf and the slender.Plant Physiol. 2024 Apr 30;195(1):111-134. doi: 10.1093/plphys/kiae044. Plant Physiol. 2024. PMID: 38290048 Free PMC article. Review.
-
The pea gene NA encodes ent-kaurenoic acid oxidase.Plant Physiol. 2003 Jan;131(1):335-44. doi: 10.1104/pp.012963. Plant Physiol. 2003. PMID: 12529541 Free PMC article.
-
Subcellular localization of fungal specialized metabolites.Fungal Biol Biotechnol. 2022 May 25;9(1):11. doi: 10.1186/s40694-022-00140-z. Fungal Biol Biotechnol. 2022. PMID: 35614515 Free PMC article. Review.
-
Characterization of the fungal gibberellin desaturase as a 2-oxoglutarate-dependent dioxygenase and its utilization for enhancing plant growth.Plant Physiol. 2012 Oct;160(2):837-45. doi: 10.1104/pp.112.201756. Epub 2012 Aug 21. Plant Physiol. 2012. PMID: 22911627 Free PMC article.
References
-
- Hooley R. Plant Mol Biol. 1994;26:1529–1555. - PubMed
-
- Rademacher W. Plant Growth Regul. 1994;15:303–314.
-
- MacMillan J. Nat Prod Res. 1997;14:221–243.
-
- Tudzynski B. In: The Mycota V, Plant Relationship, Part A. Esser K, Lemke P A, editors. Berlin: Springer; 1997. pp. 167–184.
-
- Hedden P, Phillips A L. Trends Plant Sci. 2000;5:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

