Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells
- PMID: 11312359
- PMCID: PMC114242
- DOI: 10.1128/JVI.75.10.4878-4888.2001
Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells
Abstract
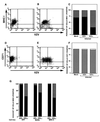
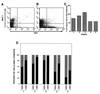
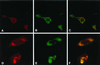
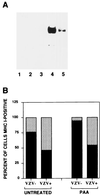
We sought to examine the effects of varicella-zoster virus (VZV) infection on the expression of major histocompatibility complex class I (MHC I) molecules by human fibroblasts and T lymphocytes. By flow cytometry, VZV infection reduced the cell surface expression of MHC I molecules on fibroblasts significantly, yet the expression of transferrin receptor was not affected. Importantly, when human fetal thymus/liver implants in SCID-hu mice were inoculated with VZV, cell surface MHC I expression was downregulated specifically on VZV-infected human CD3+ T lymphocytes, a prominent target that sustains VZV viremia. The stage in the MHC I assembly process that was disrupted by VZV in fibroblasts was examined in pulse-chase and immunoprecipitation experiments in the presence of endoglycosidase H. MHC I complexes continued to be assembled in VZV-infected cells and were not retained in the endoplasmic reticulum. In contrast, immunofluorescence and confocal microscopy showed that VZV infection resulted in an accumulation of MHC I molecules which colocalized to the Golgi compartment. Inhibition of late viral gene expression by treatment of infected fibroblasts with phosphonoacetic acid did not influence the modulation of MHC I expression, nor did transfection of cells with plasmids expressing immediate early viral proteins. However, cells transfected with a plasmid carrying the early gene ORF66 did result in a significant downregulation of MHC I expression, suggesting that this gene encodes a protein with an immunomodulatory function. Thus, VZV downregulates MHC I expression by impairing the transport of MHC I molecules from the Golgi compartment to the cell surface; this effect may enable the virus to evade CD8+ T-cell immune recognition during VZV pathogenesis, including the critical phase of T-lymphocyte-associated viremia.
Figures

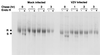

Similar articles
-
Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms.J Virol. 2007 Sep;81(17):9034-49. doi: 10.1128/JVI.00711-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567702 Free PMC article.
-
Modulation of major histocompatibility class II protein expression by varicella-zoster virus.J Virol. 2000 Feb;74(4):1900-7. doi: 10.1128/jvi.74.4.1900-1907.2000. J Virol. 2000. PMID: 10644363 Free PMC article.
-
Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens.J Infect Dis. 1998 May;177(5):1390-3. doi: 10.1086/517821. J Infect Dis. 1998. PMID: 9593031
-
Immune evasion mechanisms of varicella-zoster virus.Arch Virol Suppl. 2001;(17):99-107. doi: 10.1007/978-3-7091-6259-0_11. Arch Virol Suppl. 2001. PMID: 11339556 Review.
-
Varicella-zoster virus immune evasion.Immunol Rev. 1999 Apr;168:143-56. doi: 10.1111/j.1600-065x.1999.tb01289.x. Immunol Rev. 1999. PMID: 10399071 Review.
Cited by
-
Varicella-Zoster Virus Downregulates Programmed Death Ligand 1 and Major Histocompatibility Complex Class I in Human Brain Vascular Adventitial Fibroblasts, Perineurial Cells, and Lung Fibroblasts.J Virol. 2016 Nov 14;90(23):10527-10534. doi: 10.1128/JVI.01546-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630241 Free PMC article.
-
Varicella-zoster virus (VZV) ORF17 protein induces RNA cleavage and is critical for replication of VZV at 37 degrees C but not 33 degrees C.J Virol. 2002 Nov;76(21):11012-23. doi: 10.1128/jvi.76.21.11012-11023.2002. J Virol. 2002. PMID: 12368344 Free PMC article.
-
Varicella Zoster Virus Impairs Expression of the Nonclassical Major Histocompatibility Complex Class I-Related Gene Protein (MR1).J Infect Dis. 2023 Feb 1;227(3):391-401. doi: 10.1093/infdis/jiab526. J Infect Dis. 2023. PMID: 34648018 Free PMC article.
-
VZV infection of keratinocytes: production of cell-free infectious virions in vivo.Curr Top Microbiol Immunol. 2010;342:173-88. doi: 10.1007/82_2010_13. Curr Top Microbiol Immunol. 2010. PMID: 20225011 Free PMC article. Review.
-
Varicella-zoster virus modulates NF-kappaB recruitment on selected cellular promoters.J Virol. 2007 Dec;81(23):13092-104. doi: 10.1128/JVI.01378-07. Epub 2007 Sep 12. J Virol. 2007. PMID: 17855547 Free PMC article.
References
-
- Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P A, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. - PubMed
-
- Arvin A. Varicella-zoster virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphian, Pa: Lippincott-Raven Publishers; 1996. pp. 2547–2586.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

