Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion
- PMID: 11296224
- PMCID: PMC125420
- DOI: 10.1093/emboj/20.8.1910
Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion
Abstract
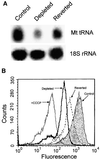
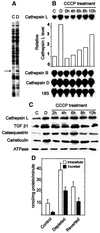
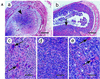
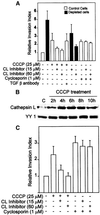
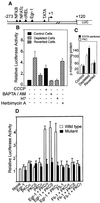
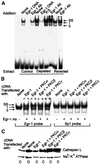
Recently we showed that partial depletion of mitochondrial DNA (genetic stress) or treatment with mitochondrial-specific inhibitors (metabolic stress) induced a stress signaling that was associated with increased cytoplasmic-free Ca(2+) [Ca(2+)](c). In the present study we show that the mitochondria-to-nucleus stress signaling induces invasive phenotypes in otherwise non-invasive C2C12 myoblasts and human pulmonary carcinoma A549 cells. Tumor-specific markers cathepsin L and transforming growth factor beta (TGFbeta) are overexpressed in cells subjected to mitochondrial genetic as well as metabolic stress. C2C12 myoblasts subjected to stress showed 4- to 6-fold higher invasion through reconstituted Matrigel membrane as well as rat tracheal xenotransplants in Scid mice. Activation of Ca(2+)-dependent protein kinase C (PKC) under both genetic and metabolic stress conditions was associated with increased cathepsin L gene expression, which contributes to increased invasive property of cells. Reverted cells with approximately 70% of control cell mtDNA exhibited marker mRNA contents, cell morphology and invasive property closer to control cells. These results provide insights into a new pathway by which mitochondrial DNA and membrane damage can contribute to tumor progression and metastasis.
Figures

Similar articles
-
Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells.Oncogene. 2002 Nov 7;21(51):7839-49. doi: 10.1038/sj.onc.1205983. Oncogene. 2002. PMID: 12420221
-
Dioxin-mediated tumor progression through activation of mitochondria-to-nucleus stress signaling.Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):186-91. doi: 10.1073/pnas.0706183104. Epub 2008 Jan 2. Proc Natl Acad Sci U S A. 2008. PMID: 18172213 Free PMC article.
-
Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis.Gene. 2005 Jul 18;354:132-9. doi: 10.1016/j.gene.2005.03.028. Gene. 2005. PMID: 15978749 Free PMC article.
-
The role of cathepsin L in malignant transformation.Semin Cancer Biol. 1990 Apr;1(2):127-36. Semin Cancer Biol. 1990. PMID: 2103489 Review.
-
Lysosomal cysteine peptidases - Molecules signaling tumor cell death and survival.Semin Cancer Biol. 2015 Dec;35:168-79. doi: 10.1016/j.semcancer.2015.08.001. Epub 2015 Aug 7. Semin Cancer Biol. 2015. PMID: 26255843 Review.
Cited by
-
Mitochondria and cancer: past, present, and future.Biomed Res Int. 2013;2013:612369. doi: 10.1155/2013/612369. Epub 2013 Jan 28. Biomed Res Int. 2013. PMID: 23509753 Free PMC article. Review.
-
Mitochondrial D310 mutations in the early development of breast cancer.Br J Cancer. 2012 Apr 24;106(9):1506-11. doi: 10.1038/bjc.2012.74. Br J Cancer. 2012. PMID: 22472881 Free PMC article.
-
Mitochondrial retrograde signaling in the Drosophila nervous system and beyond.Fly (Austin). 2016 Jan 2;10(1):19-24. doi: 10.1080/19336934.2016.1174353. Epub 2016 Apr 11. Fly (Austin). 2016. PMID: 27064199 Free PMC article.
-
Mitochondrial regulation of cancer associated nuclear DNA methylation.Biochem Biophys Res Commun. 2007 Dec 21;364(3):656-61. doi: 10.1016/j.bbrc.2007.10.047. Epub 2007 Oct 16. Biochem Biophys Res Commun. 2007. PMID: 17964537 Free PMC article.
-
hnRNPA2 mediated acetylation reduces telomere length in response to mitochondrial dysfunction.PLoS One. 2018 Nov 14;13(11):e0206897. doi: 10.1371/journal.pone.0206897. eCollection 2018. PLoS One. 2018. PMID: 30427907 Free PMC article.
References
-
- Allen J.A. and Coombs,M.M. (1980) Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature, 287, 244–245. - PubMed
-
- Atkins K.B. and Troen,B.R. (1995) Phorbol ester stimulated cathepsin L expression in U937 cells. Cell Growth Differ., 6, 713–718. - PubMed
-
- Backer J.M. and Weinstein,I.B. (1980) Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science, 209, 297–299. - PubMed
-
- Biswas G., Adebanjo,O.A., Freedman,B.D., Anandatheerthavarada, H.K., Vijayasarathy,C., Zaidi,M., Kotlikoff,M. and Avadhani,N.G. (1999) Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J., 18, 522–533. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

