Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice
- PMID: 11290559
- PMCID: PMC1891895
- DOI: 10.1016/s0002-9440(10)64092-8
Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice
Abstract
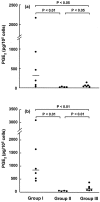
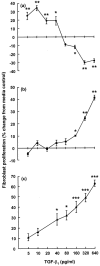
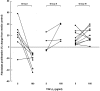
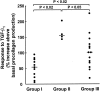
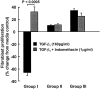
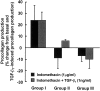
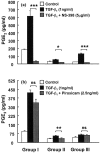
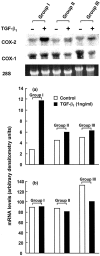
Prostaglandin E(2) (PGE(2)) inhibits fibroblast proliferation and collagen production. Its synthesis by fibroblasts is induced by profibrotic mediators including transforming growth factor (TGF)-beta(1). However, in patients with pulmonary fibrosis, PGE(2) levels are decreased. In this study we examined the effect of TGF-beta(1) on PGE(2) synthesis, proliferation, collagen production, and cyclooxygenase (COX) mRNA levels in fibroblasts derived from fibrotic and nonfibrotic human lung. In addition, we examined the effect of bleomycin-induced pulmonary fibrosis in COX-2-deficient mice. We demonstrate that basal and TGF-beta(1)-induced PGE(2) synthesis is limited in fibroblasts from fibrotic lung. Functionally, this correlates with a loss of the anti-proliferative response to TGF-beta(1). This failure to induce PGE(2) synthesis is because of an inability to up-regulate COX-2 mRNA levels in these fibroblasts. Furthermore, mice deficient in COX-2 exhibit an enhanced response to bleomycin. We conclude that a decreased capacity to up-regulate COX-2 expression and COX-2-derived PGE(2) synthesis in the presence of increasing levels of profibrotic mediators such as TGF-beta(1) may lead to unopposed fibroblast proliferation and collagen synthesis and contribute to the pathogenesis of pulmonary fibrosis.
Figures

Similar articles
-
Prostaglandin E2 inhibits transforming growth factor beta 1-mediated induction of collagen alpha 1(I) in hepatic stellate cells.J Hepatol. 2004 Aug;41(2):251-8. doi: 10.1016/j.jhep.2004.04.033. J Hepatol. 2004. PMID: 15288474
-
Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production.Am J Pathol. 2004 Nov;165(5):1663-76. doi: 10.1016/S0002-9440(10)63423-2. Am J Pathol. 2004. PMID: 15509536 Free PMC article.
-
Both cyclooxygenase-1 and cyclooxygenase-2 mediate osteoblast response to titanium surface roughness.J Biomed Mater Res. 2001 Jun 5;55(3):350-9. doi: 10.1002/1097-4636(20010605)55:3<350::aid-jbm1023>3.0.co;2-m. J Biomed Mater Res. 2001. PMID: 11255188
-
Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis.Am J Pathol. 1997 Mar;150(3):981-91. Am J Pathol. 1997. PMID: 9060836 Free PMC article.
-
Prostaglandin E2 and the pathogenesis of pulmonary fibrosis.Am J Respir Cell Mol Biol. 2011 Sep;45(3):445-52. doi: 10.1165/rcmb.2011-0025RT. Epub 2011 Mar 18. Am J Respir Cell Mol Biol. 2011. PMID: 21421906 Free PMC article. Review.
Cited by
-
Prostaglandin Transporter (PGT/SLCO2A1) Protects the Lung from Bleomycin-Induced Fibrosis.PLoS One. 2015 Apr 29;10(4):e0123895. doi: 10.1371/journal.pone.0123895. eCollection 2015. PLoS One. 2015. PMID: 25923111 Free PMC article.
-
Transforming Growth Factor-β1 Increases DNA Methyltransferase 1 and 3a Expression through Distinct Post-transcriptional Mechanisms in Lung Fibroblasts.J Biol Chem. 2016 Sep 9;291(37):19287-98. doi: 10.1074/jbc.M116.723080. Epub 2016 Jul 12. J Biol Chem. 2016. PMID: 27405758 Free PMC article.
-
Prostaglandin E2 mediates IL-1beta-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors.J Immunol. 2008 Jan 1;180(1):637-46. doi: 10.4049/jimmunol.180.1.637. J Immunol. 2008. PMID: 18097066 Free PMC article.
-
Crystalline and amorphous silica differentially regulate the cyclooxygenase-prostaglandin pathway in pulmonary fibroblasts: implications for pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2005 Jun;288(6):L1010-6. doi: 10.1152/ajplung.00024.2004. Epub 2005 Jan 21. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15665045 Free PMC article.
-
Modulation of COX-2 and NADPH oxidase-4 by alpha-lipoic acid ameliorates busulfan-induced pulmonary injury in rats.Heliyon. 2021 Oct 13;7(10):e08171. doi: 10.1016/j.heliyon.2021.e08171. eCollection 2021 Oct. Heliyon. 2021. PMID: 34746462 Free PMC article.
References
-
- Harrison NK, Myers AR, Corrin B, Soosay G, Dewar A, Black CM, Du Bois RM, Turner-Warwick M: Structural features of interstitial lung disease in systemic sclerosis. Am Rev Respir Dis 1991, 144:706-713 - PubMed
-
- Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, Lake FR: Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997, 156:528-535 - PubMed
-
- McAnulty RJ, Laurent GJ: Collagen and its regulation in pulmonary fibrosis. Phan SH Thrall RS eds. Pulmonary Fibrosis. 1995, :pp 135-171 Marcel Dekker, New York
-
- Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ: A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci 1989, 92:513-518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

