Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits
- PMID: 11287600
- PMCID: PMC114196
- DOI: 10.1128/JVI.75.9.4453-4458.2001
Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits
Abstract
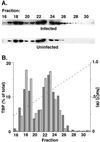
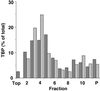
The matrix (M) protein of vesicular stomatitis virus (VSV) is a potent inhibitor in vivo of transcription by all three host RNA polymerases (RNAP). In the case of host RNA polymerase II (RNAPII), the inhibition is due to lack of activity of the TATA-binding protein (TBP), which is a subunit of the basal transcription factor TFIID. Despite the potency of M protein-induced inhibition in vivo, experiments presented here show that M protein cannot directly inactivate TFIID in vitro. Addition of M protein to nuclear extracts from uninfected cells did not inhibit transcription activity, indicating that the inhibition is indirect and is mediated through host factors. The host factors that are known to regulate TBP activity include phosphorylation by host kinases and association with different TBP-associated factor (TAF) subunits. However, TBP in VSV-infected cells was found to be assembled normally with its TAF subunits, as shown by ion exchange high-pressure liquid chromatography and sedimentation velocity analysis. A normal pattern of phosphorylation of TBP in VSV-infected cells was also observed by pH gradient gel electrophoresis. Collectively, these data indicate that M protein inactivates TBP activity in RNAPII-dependent transcription by a novel mechanism, since the known mechanisms for regulating TBP activity cannot account for the inhibition.
Figures


Similar articles
-
Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID.Virology. 1998 Nov 25;251(2):383-92. doi: 10.1006/viro.1998.9413. Virology. 1998. PMID: 9837802
-
TAF(II)170 interacts with the concave surface of TATA-binding protein to inhibit its DNA binding activity.Mol Cell Biol. 2001 Nov;21(21):7523-34. doi: 10.1128/MCB.21.21.7523-7534.2001. Mol Cell Biol. 2001. PMID: 11585931 Free PMC article.
-
Chromatin is permissive to TATA-binding protein (TBP)-mediated transcription initiation.J Biol Chem. 2001 Apr 20;276(16):12781-4. doi: 10.1074/jbc.M100481200. Epub 2001 Jan 22. J Biol Chem. 2001. PMID: 11279078
-
Mechanisms of transcriptional activation and repression can both involve TFIID.Philos Trans R Soc Lond B Biol Sci. 1996 Apr 29;351(1339):517-26. doi: 10.1098/rstb.1996.0050. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8735274 Review.
-
Retinoid-dependent transcription: the RAR/RXR-TBP-EIA/EIA-LA connection.Biochem Soc Symp. 1996;62:97-109. Biochem Soc Symp. 1996. PMID: 8971343 Review.
Cited by
-
Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication.PLoS One. 2008 Apr 2;3(4):e1887. doi: 10.1371/journal.pone.0001887. PLoS One. 2008. PMID: 18382670 Free PMC article.
-
NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription.J Virol. 2004 Sep;78(18):9798-806. doi: 10.1128/JVI.78.18.9798-9806.2004. J Virol. 2004. PMID: 15331713 Free PMC article.
-
The role of the influenza virus RNA polymerase in host shut-off.Virulence. 2010 Sep-Oct;1(5):436-9. doi: 10.4161/viru.1.5.12967. Virulence. 2010. PMID: 21178485 Free PMC article. Review.
-
De novo assembly and transcriptome analysis of Atlantic salmon macrophage/dendritic-like TO cells following type I IFN treatment and Salmonid alphavirus subtype-3 infection.BMC Genomics. 2015 Feb 18;16(1):96. doi: 10.1186/s12864-015-1302-1. BMC Genomics. 2015. PMID: 25765343 Free PMC article.
-
The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death.J Virol. 2003 May;77(9):5524-8. doi: 10.1128/jvi.77.9.5524-5528.2003. J Virol. 2003. PMID: 12692256 Free PMC article.
References
-
- Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

