Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase
- PMID: 11274421
- PMCID: PMC31166
- DOI: 10.1073/pnas.071050898
Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase
Abstract
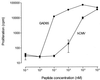
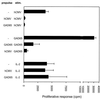
Antigens of pathogenic microbes that mimic autoantigens are thought to be responsible for the activation of autoreactive T cells. Viral infections have been associated with the development of the neuroendocrine autoimmune diseases type 1 diabetes and stiff-man syndrome, but the mechanism is unknown. These diseases share glutamic acid decarboxylase (GAD65) as a major autoantigen. We screened synthetic peptide libraries dedicated to bind to HLA-DR3, which predisposes to both diseases, using clonal CD4(+) T cells reactive to GAD65 isolated from a prediabetic stiff-man syndrome patient. Here we show that these GAD65-specific T cells crossreact with a peptide of the human cytomegalovirus (hCMV) major DNA-binding protein. This peptide was identified after database searching with a recognition pattern that had been deduced from the library studies. Furthermore, we showed that hCMV-derived epitope can be naturally processed by dendritic cells and recognized by GAD65 reactive T cells. Thus, hCMV may be involved in the loss of T cell tolerance to autoantigen GAD65 by a mechanism of molecular mimicry leading to autoimmunity.
Figures

Similar articles
-
Molecular mimicry in type 1 diabetes: immune cross-reactivity between islet autoantigen and human cytomegalovirus but not Coxsackie virus.Ann N Y Acad Sci. 2002 Apr;958:163-5. Ann N Y Acad Sci. 2002. PMID: 12021098
-
GAD65-Reactive T cells in a non-diabetic stiff-man syndrome patient.J Autoimmun. 1999 Jun;12(4):289-96. doi: 10.1006/jaut.1999.0280. J Autoimmun. 1999. PMID: 10330300
-
Differential presentation of glutamic acid decarboxylase 65 (GAD65) T cell epitopes among HLA-DRB1*0401-positive individuals.J Immunol. 1999 Aug 1;163(3):1674-81. J Immunol. 1999. PMID: 10415074
-
Autoimmune mechanisms in type 1 diabetes.Autoimmun Rev. 2008 Jul;7(7):550-7. doi: 10.1016/j.autrev.2008.04.008. Epub 2008 Apr 30. Autoimmun Rev. 2008. PMID: 18625444 Review.
-
[Molecular mimicry and mechanisms of autoantibody production].Nihon Rinsho. 1997 Jun;55(6):1370-6. Nihon Rinsho. 1997. PMID: 9200920 Review. Japanese.
Cited by
-
Beta-cell Specific Autoantibodies: Are they Just an Indicator of Type 1 Diabetes?Curr Diabetes Rev. 2017;13(3):322-329. doi: 10.2174/1573399812666160427104157. Curr Diabetes Rev. 2017. PMID: 27117244 Free PMC article. Review.
-
β cell ER stress and the implications for immunogenicity in type 1 diabetes.Front Cell Dev Biol. 2015 Oct 27;3:67. doi: 10.3389/fcell.2015.00067. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26579520 Free PMC article. Review.
-
Environmental Factors Contribute to β Cell Endoplasmic Reticulum Stress and Neo-Antigen Formation in Type 1 Diabetes.Front Endocrinol (Lausanne). 2017 Sep 29;8:262. doi: 10.3389/fendo.2017.00262. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29033899 Free PMC article.
-
New onset diabetes, type 1 diabetes and COVID-19.Diabetes Metab Syndr. 2020 Nov-Dec;14(6):2211-2217. doi: 10.1016/j.dsx.2020.11.012. Epub 2020 Nov 17. Diabetes Metab Syndr. 2020. PMID: 33395782 Free PMC article. Review.
-
Autoreactive T cells in type 1 diabetes.J Clin Invest. 2017 Aug 1;127(8):2881-2891. doi: 10.1172/JCI94549. Epub 2017 Aug 1. J Clin Invest. 2017. PMID: 28762987 Free PMC article. Review.
References
-
- Baekkeskov S, Aanstoot H J, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter Olesen H, DeCamilli P. Nature (London) 1990;347:151–156. - PubMed
-
- Ellis T M, Atkinson M A. Nat Med. 1996;2:148–153. - PubMed
-
- Roep B O. Diabetes. 1996;45:1147–1156. - PubMed
-
- Lorish T R, Thorsteinsson G, Howard F M. Mayo Clin Proc. 1989;64:629–636. - PubMed
-
- Solimena M, Folli F, Denis-Donini S, Comi G C, Pozza G, DeCamilli P, Vicari A M. N Engl J Med. 1988;318:1012–1020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

