Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9
- PMID: 11264375
- PMCID: PMC114877
- DOI: 10.1128/JVI.75.8.3859-3872.2001
Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9
Abstract
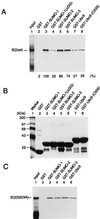
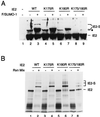
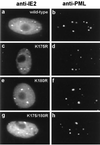
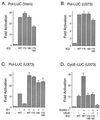
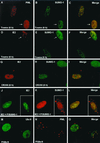
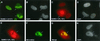
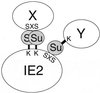
The human cytomegalovirus (HCMV) major immediate-early protein IE2 is a nuclear phosphoprotein that is believed to be a key regulator in both lytic and latent infections. Using yeast two-hybrid screening, small ubiquitin-like modifiers (SUMO-1, SUMO-2, and SUMO-3) and a SUMO-conjugating enzyme (Ubc9) were isolated as IE2-interacting proteins. In vitro binding assays with glutathione S-transferase (GST) fusion proteins provided evidence for direct protein-protein interaction. Mapping data showed that the C-terminal end of SUMO-1 is critical for interaction with IE2 in both yeast and in vitro binding assays. IE2 was efficiently modified by SUMO-1 or SUMO-2 in cotransfected cells and in cells infected with a recombinant adenovirus expressing HCMV IE2, although the level of modification was much lower in HCMV-infected cells. Two lysine residues at positions 175 and 180 were mapped as major alternative SUMO-1 conjugation sites in both cotransfected cells and an in vitro sumoylation assay and could be conjugated by SUMO-1 simultaneously. Although mutations of these lysine residues did not interfere with the POD (or ND10) targeting of IE2, overexpression of SUMO-1 enhanced IE2-mediated transactivation in a promoter-dependent manner in reporter assays. Interestingly, many other cellular proteins identified as IE2 interaction partners in yeast two-hybrid assays also interact with SUMO-1, suggesting that either directly bound or covalently conjugated SUMO moieties may act as a bridge for interactions between IE2 and other SUMO-1-modified or SUMO-1-interacting proteins. When we investigated the intracellular localization of SUMO-1 in HCMV-infected cells, the pattern changed from nuclear punctate to predominantly nuclear diffuse in an IE1-dependent manner at very early times after infection, but with some SUMO-1 protein now associated with IE2 punctate domains. However, at late times after infection, SUMO-1 was predominantly detected within viral DNA replication compartments containing IE2. Taken together, these results show that HCMV infection causes the redistribution of SUMO-1 and that IE2 both physically binds to and is covalently modified by SUMO moieties, suggesting possible modulation of both the function of SUMO-1 and protein-protein interactions of IE2 during HCMV infection.
Figures



Similar articles
-
Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b.J Virol. 2000 Mar;74(6):2510-24. doi: 10.1128/jvi.74.6.2510-2524.2000. J Virol. 2000. PMID: 10684265 Free PMC article.
-
The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10).J Virol. 1999 Dec;73(12):10458-71. doi: 10.1128/JVI.73.12.10458-10471.1999. J Virol. 1999. PMID: 10559364 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells.J Virol. 1997 Jun;71(6):4599-613. doi: 10.1128/JVI.71.6.4599-4613.1997. J Virol. 1997. PMID: 9151854 Free PMC article.
-
Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression.J Virol. 2001 Nov;75(22):10683-95. doi: 10.1128/JVI.75.22.10683-10695.2001. J Virol. 2001. PMID: 11602710 Free PMC article.
Cited by
-
Repression of HMGA2 gene expression by human cytomegalovirus involves the IE2 86-kilodalton protein and is necessary for efficient viral replication and inhibition of cyclin A transcription.J Virol. 2006 Oct;80(20):9951-61. doi: 10.1128/JVI.01300-06. J Virol. 2006. PMID: 17005673 Free PMC article.
-
Internal deletions of IE2 86 and loss of the late IE2 60 and IE2 40 proteins encoded by human cytomegalovirus affect the levels of UL84 protein but not the amount of UL84 mRNA or the loading and distribution of the mRNA on polysomes.J Virol. 2008 Nov;82(22):11383-97. doi: 10.1128/JVI.01293-08. Epub 2008 Sep 10. J Virol. 2008. PMID: 18787008 Free PMC article.
-
Sumoylation at the host-pathogen interface.Biomolecules. 2012 Apr 5;2(2):203-27. doi: 10.3390/biom2020203. Biomolecules. 2012. PMID: 23795346 Free PMC article.
-
Functional interaction between human herpesvirus 6 immediate-early 2 protein and ubiquitin-conjugating enzyme 9 in the absence of sumoylation.J Virol. 2006 Oct;80(20):10218-28. doi: 10.1128/JVI.00375-06. J Virol. 2006. PMID: 17005699 Free PMC article.
-
SUMO modifies GβL and mediates mTOR signaling.J Biol Chem. 2024 Apr;300(4):105778. doi: 10.1016/j.jbc.2024.105778. Epub 2024 Feb 21. J Biol Chem. 2024. PMID: 38395307 Free PMC article.
References
-
- Ahn J H, Chiou C J, Hayward G S. Evaluation and mapping of the DNA binding and oligomerization domains of the IE2 regulatory protein of human cytomegalovirus using yeast one and two hybrid interaction assays. Gene. 1998;210:25–36. - PubMed
-
- Ahn J H, Hayward G S. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology. 2000;274:39–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

