S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3
- PMID: 11259591
- PMCID: PMC86875
- DOI: 10.1128/MCB.21.7.2423-2434.2001
S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3
Abstract
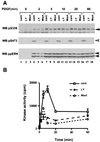
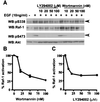
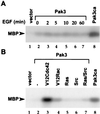
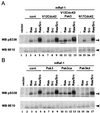
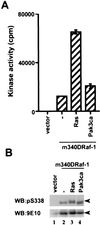
The Raf-1 serine/threonine protein kinase requires phosphorylation of the serine at position 338 (S338) for activation. Ras is required to recruit Raf-1 to the plasma membrane, which is where S338 phosphorylation occurs. The recent suggestion that Pak3 could stimulate Raf-1 activity by directly phosphorylating S338 through a Ras/phosphatidylinositol 3-kinase (Pl3-K)/-Cdc42-dependent pathway has attracted much attention. Using a phospho-specific antibody to S338, we have reexamined this model. Using LY294002 and wortmannin, inhibitors of Pl3-K, we find that growth factor-mediated S338 phosphorylation still occurs, even when Pl3-K activity is completely blocked. Although high concentrations of LY294002 and wortmannin did suppress S338 phosphorylation, they also suppressed Ras activation. Additionally, we show that Pak3 is not activated under conditions where S338 is phosphorylated, but when Pak3 is strongly activated, by coexpression with V12Cdc42 or by mutations that make it independent of Cdc42, it did stimulate S338 phosphorylation. However, this occurred in the cytosol and did not stimulate Raf-1 kinase activity. The inability of Pak3 to activate Raf-1 was not due to an inability to stimulate phosphorylation of the tyrosine at position 341 but may be due to its inability to recruit Raf-1 to the plasma membrane. Taken together, our data show that growth factor-stimulated Raf-1 activity is independent of Pl3-K activity and argue against Pak3 being a physiological mediator of S338 phosphorylation in growth factor-stimulated cells.
Figures




Similar articles
-
Calcium/calmodulin-dependent protein kinase II (CaMKII) phosphorylates Raf-1 at serine 338 and mediates Ras-stimulated Raf-1 activation.Cell Cycle. 2012 Jun 1;11(11):2100-6. doi: 10.4161/cc.20543. Epub 2012 Jun 1. Cell Cycle. 2012. PMID: 22592532
-
Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak.Curr Biol. 2000 Mar 9;10(5):281-4. doi: 10.1016/s0960-9822(00)00359-6. Curr Biol. 2000. PMID: 10712905
-
The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338.Nature. 1998 Nov 12;396(6707):180-3. doi: 10.1038/24184. Nature. 1998. PMID: 9823899
-
Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling.Mol Cell Biol. 1997 Aug;17(8):4509-16. doi: 10.1128/MCB.17.8.4509. Mol Cell Biol. 1997. PMID: 9234708 Free PMC article.
-
Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade.Recent Prog Horm Res. 2001;56:127-55. doi: 10.1210/rp.56.1.127. Recent Prog Horm Res. 2001. PMID: 11237210 Review.
Cited by
-
14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity.Mol Cell Biol. 2002 Jul;22(14):4984-96. doi: 10.1128/MCB.22.14.4984-4996.2002. Mol Cell Biol. 2002. PMID: 12077328 Free PMC article.
-
Targeting prostate cancer based on signal transduction and cell cycle pathways.Cell Cycle. 2008 Jun 15;7(12):1745-62. doi: 10.4161/cc.7.12.6166. Epub 2008 Jun 16. Cell Cycle. 2008. PMID: 18594202 Free PMC article. Review.
-
Ras-Mediated Activation of the Raf Family Kinases.Cold Spring Harb Perspect Med. 2019 Jan 2;9(1):a033746. doi: 10.1101/cshperspect.a033746. Cold Spring Harb Perspect Med. 2019. PMID: 29358316 Free PMC article. Review.
-
The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression.Pharmacol Ther. 2007 Jan;113(1):88-120. doi: 10.1016/j.pharmthera.2006.07.004. Epub 2006 Nov 13. Pharmacol Ther. 2007. PMID: 17097148 Free PMC article. Review.
-
Regulation of Raf-1 activation and signalling by dephosphorylation.EMBO J. 2002 Jan 15;21(1-2):64-71. doi: 10.1093/emboj/21.1.64. EMBO J. 2002. PMID: 11782426 Free PMC article.
References
-
- Bagrodia S, Cerione R A. PAK to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
-
- Barnard D, Diaz B, Clawson D, Marshall M. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene. 1998;17:1539–1547. - PubMed
-
- Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
