Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication
- PMID: 11257126
- PMCID: PMC2199215
- DOI: 10.1083/jcb.152.6.1267
Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication
Abstract
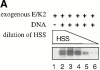
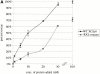
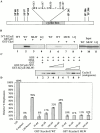
Using an in vitro chromatin assembly assay in Xenopus egg extract, we show that cyclin E binds specifically and saturably to chromatin in three phases. In the first phase, the origin recognition complex and Cdc6 prereplication proteins, but not the minichromosome maintenance complex, are necessary and biochemically sufficient for ATP-dependent binding of cyclin E--Cdk2 to DNA. We find that cyclin E binds the NH(2)-terminal region of Cdc6 containing Cy--Arg-X-Leu (RXL) motifs. Cyclin E proteins with mutated substrate selection (Met-Arg-Ala-Ile-Leu; MRAIL) motifs fail to bind Cdc6, fail to compete with endogenous cyclin E--Cdk2 for chromatin binding, and fail to rescue replication in cyclin E--depleted extracts. Cdc6 proteins with mutations in the three consensus RXL motifs are quantitatively deficient for cyclin E binding and for rescuing replication in Cdc6-depleted extracts. Thus, the cyclin E--Cdc6 interaction that localizes the Cdk2 complex to chromatin is important for DNA replication. During the second phase, cyclin E--Cdk2 accumulates on chromatin, dependent on polymerase activity. In the third phase, cyclin E is phosphorylated, and the cyclin E--Cdk2 complex is displaced from chromatin in mitosis. In vitro, mitogen-activated protein kinase and especially cyclin B--Cdc2, but not the polo-like kinase 1, remove cyclin E--Cdk2 from chromatin. Rebinding of hyperphosphorylated cyclin E--Cdk2 to interphase chromatin requires dephosphorylation, and the Cdk kinase-directed Cdc14 phosphatase is sufficient for this dephosphorylation in vitro. These three phases of cyclin E association with chromatin may facilitate the diverse activities of cyclin E--Cdk2 in initiating replication, blocking rereplication, and allowing resetting of origins after mitosis.
Figures








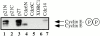






Similar articles
-
Triggering ubiquitination of a CDK inhibitor at origins of DNA replication.Nat Cell Biol. 2001 Aug;3(8):715-22. doi: 10.1038/35087026. Nat Cell Biol. 2001. PMID: 11483956
-
Cyclin A-dependent kinase activity affects chromatin binding of ORC, Cdc6, and MCM in egg extracts of Xenopus laevis.Eur J Biochem. 1999 Sep;264(2):415-26. doi: 10.1046/j.1432-1327.1999.00613.x. Eur J Biochem. 1999. PMID: 10491086
-
Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2.J Cell Biol. 1998 Jan 26;140(2):271-81. doi: 10.1083/jcb.140.2.271. J Cell Biol. 1998. PMID: 9442103 Free PMC article.
-
Cyclin-dependent kinases and S phase control in mammalian cells.Cell Cycle. 2003 Jul-Aug;2(4):316-24. Cell Cycle. 2003. PMID: 12851482 Review.
-
Control of DNA replication licensing in a cell cycle.Genes Cells. 2002 Jun;7(6):523-34. doi: 10.1046/j.1365-2443.2002.00544.x. Genes Cells. 2002. PMID: 12059957 Review.
Cited by
-
The cyclin A centrosomal localization sequence recruits MCM5 and Orc1 to regulate centrosome reduplication.J Cell Sci. 2010 Aug 15;123(Pt 16):2743-9. doi: 10.1242/jcs.073098. Epub 2010 Jul 27. J Cell Sci. 2010. PMID: 20663915 Free PMC article.
-
Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity.Mol Cell Biol. 2005 Apr;25(8):3364-87. doi: 10.1128/MCB.25.8.3364-3387.2005. Mol Cell Biol. 2005. PMID: 15798220 Free PMC article.
-
Wee1 kinase alters cyclin E/Cdk2 and promotes apoptosis during the early embryonic development of Xenopus laevis.BMC Dev Biol. 2007 Oct 25;7:119. doi: 10.1186/1471-213X-7-119. BMC Dev Biol. 2007. PMID: 17961226 Free PMC article.
-
Binding of cyclin-dependent kinases to ORC and Cdc6p regulates the chromosome replication cycle.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11211-7. doi: 10.1073/pnas.201387198. Proc Natl Acad Sci U S A. 2001. PMID: 11572976 Free PMC article.
-
Grouped graphical Granger modeling for gene expression regulatory networks discovery.Bioinformatics. 2009 Jun 15;25(12):i110-8. doi: 10.1093/bioinformatics/btp199. Bioinformatics. 2009. PMID: 19477976 Free PMC article.
References
-
- Bell S.P., Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. - PubMed
-
- Carpenter P.B., Mueller P.R., Dunphy W.G. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–360. - PubMed
-
- Chen J., Jackson P.K., Kirschner M.W., Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

