The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells
- PMID: 11257120
- PMCID: PMC2199202
- DOI: 10.1083/jcb.152.6.1197
The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells
Abstract
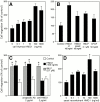
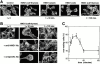
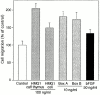
HMG1 (high mobility group 1) is a ubiquitous and abundant chromatin component. However, HMG1 can be secreted by activated macrophages and monocytes, and can act as a mediator of inflammation and endotoxic lethality. Here we document a role of extracellular HMG1 in cell migration. HMG1 (and its individual DNA-binding domains) stimulated migration of rat smooth muscle cells in chemotaxis, chemokinesis, and wound healing assays. HMG1 induced rapid and transient changes of cell shape, and actin cytoskeleton reorganization leading to an elongated polarized morphology typical of motile cells. These effects were inhibited by antibodies directed against the receptor of advanced glycation endproducts, indicating that the receptor of advanced glycation endproducts is the receptor mediating the HMG1-dependent migratory responses. Pertussis toxin and the mitogen-activated protein kinase kinase inhibitor PD98059 also blocked HMG1-induced rat smooth muscle cell migration, suggesting that a G(i/o) protein and mitogen-activated protein kinases are required for the HMG1 signaling pathway. We also show that HMG1 can be released by damage or necrosis of a variety of cell types, including endothelial cells. Thus, HMG1 has all the hallmarks of a molecule that can promote atherosclerosis and restenosis after vascular damage.
Figures

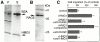


Similar articles
-
Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells.Blood. 1999 Jul 15;94(2):649-62. Blood. 1999. PMID: 10397732
-
Urokinase/urokinase receptor and vitronectin/alpha(v)beta(3) integrin induce chemotaxis and cytoskeleton reorganization through different signaling pathways.Oncogene. 2001 Apr 12;20(16):2032-43. doi: 10.1038/sj.onc.1204261. Oncogene. 2001. PMID: 11360187
-
TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent.Hypertension. 1999 Jan;33(1 Pt 2):183-9. doi: 10.1161/01.hyp.33.1.183. Hypertension. 1999. PMID: 9931102
-
Monocyte chemotactic protein 1 amplifies serotonin-induced vascular smooth muscle cell proliferation.J Vasc Res. 2001 Jul-Aug;38(4):341-9. doi: 10.1159/000051065. J Vasc Res. 2001. PMID: 11455205
-
Eukaryotic chemotaxis at a glance.J Cell Sci. 2008 Aug 15;121(Pt 16):2621-4. doi: 10.1242/jcs.018077. J Cell Sci. 2008. PMID: 18685153 Free PMC article. Review. No abstract available.
Cited by
-
Calcium/Calmodulin-Dependent Protein Kinase is Involved in the Release of High Mobility Group Box 1 Via the Interferon-β Signaling Pathway.Immune Netw. 2012 Aug;12(4):148-54. doi: 10.4110/in.2012.12.4.148. Epub 2012 Aug 31. Immune Netw. 2012. PMID: 23091438 Free PMC article.
-
Innate immune regulation by toll-like receptors in the brain.ISRN Neurol. 2012;2012:701950. doi: 10.5402/2012/701950. Epub 2012 Oct 14. ISRN Neurol. 2012. PMID: 23097717 Free PMC article.
-
Oxidative Stress and High-Mobility Group Box 1 Assay in Dogs with Gastrointestinal Parasites.Antioxidants (Basel). 2022 Aug 28;11(9):1679. doi: 10.3390/antiox11091679. Antioxidants (Basel). 2022. PMID: 36139753 Free PMC article.
-
High-mobility group box 1 inhibits HCO(3)(-) absorption in medullary thick ascending limb through a basolateral receptor for advanced glycation end products pathway.Am J Physiol Renal Physiol. 2015 Oct 15;309(8):F720-30. doi: 10.1152/ajprenal.00227.2015. Epub 2015 Jul 15. Am J Physiol Renal Physiol. 2015. PMID: 26180239 Free PMC article.
-
Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation.J Cell Biol. 2004 Feb 2;164(3):441-9. doi: 10.1083/jcb.200304135. Epub 2004 Jan 26. J Cell Biol. 2004. PMID: 14744997 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

