Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism
- PMID: 11257115
- PMCID: PMC2199204
- DOI: 10.1083/jcb.152.6.1135
Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism
Abstract
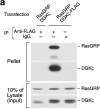
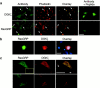
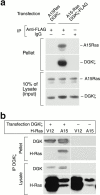
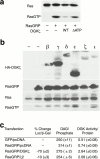
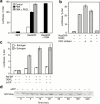
Guanine nucleotide exchange factors (GEFs) activate Ras by facilitating its GTP binding. Ras guanyl nucleotide-releasing protein (GRP) was recently identified as a Ras GEF that has a diacylglycerol (DAG)-binding C1 domain. Its exchange factor activity is regulated by local availability of signaling DAG. DAG kinases (DGKs) metabolize DAG by converting it to phosphatidic acid. Because they can attenuate local accumulation of signaling DAG, DGKs may regulate RasGRP activity and, consequently, activation of Ras. DGK zeta, but not other DGKs, completely eliminated Ras activation induced by RasGRP, and DGK activity was required for this mechanism. DGK zeta also coimmunoprecipitated and colocalized with RasGRP, indicating that these proteins associate in a signaling complex. Coimmunoprecipitation of DGK zeta and RasGRP was enhanced in the presence of phorbol esters, which are DAG analogues that cannot be metabolized by DGKs, suggesting that DAG signaling can induce their interaction. Finally, overexpression of kinase-dead DGK zeta in Jurkat cells prolonged Ras activation after ligation of the T cell receptor. Thus, we have identified a novel way to regulate Ras activation: through DGK zeta, which controls local accumulation of DAG that would otherwise activate RasGRP.
Figures



Similar articles
-
T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation.J Immunol. 2003 Mar 15;170(6):2877-83. doi: 10.4049/jimmunol.170.6.2877. J Immunol. 2003. PMID: 12626538
-
[RasGRP proteins--Ras-activating factors].Postepy Biochem. 2007;53(2):112-20. Postepy Biochem. 2007. PMID: 17969871 Review. Polish.
-
Protein kinase C alpha phosphorylates and negatively regulates diacylglycerol kinase zeta.J Biol Chem. 2003 Oct 10;278(41):39542-7. doi: 10.1074/jbc.M307153200. Epub 2003 Jul 30. J Biol Chem. 2003. PMID: 12890670
-
A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells.Mol Cell Biol. 2005 Jun;25(11):4426-41. doi: 10.1128/MCB.25.11.4426-4441.2005. Mol Cell Biol. 2005. PMID: 15899849 Free PMC article.
-
Diacylglycerol kinase is a keystone regulator of signaling relevant to the pathophysiology of asthma.Am J Physiol Lung Cell Mol Physiol. 2024 Jul 1;327(1):L3-L18. doi: 10.1152/ajplung.00091.2024. Epub 2024 May 14. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38742284 Review.
Cited by
-
Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI.Mol Biol Cell. 2009 Apr;20(7):2049-59. doi: 10.1091/mbc.e07-12-1248. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211846 Free PMC article.
-
Diacylglycerol kinases in immune cell function and self-tolerance.Immunol Rev. 2008 Aug;224:249-64. doi: 10.1111/j.1600-065X.2008.00647.x. Immunol Rev. 2008. PMID: 18759932 Free PMC article. Review.
-
Exploring the multifaceted role of RASGRP1 in disease: immune, neural, metabolic, and oncogenic perspectives.Cell Cycle. 2024 Mar;23(6):722-746. doi: 10.1080/15384101.2024.2366009. Epub 2024 Jun 12. Cell Cycle. 2024. PMID: 38865342 Review.
-
A novel IRS-1-associated protein, DGKζ regulates GLUT4 translocation in 3T3-L1 adipocytes.Sci Rep. 2016 Oct 14;6:35438. doi: 10.1038/srep35438. Sci Rep. 2016. PMID: 27739494 Free PMC article.
-
Regulation of Lipid Signaling by Diacylglycerol Kinases during T Cell Development and Function.Front Immunol. 2013 Jul 4;4:178. doi: 10.3389/fimmu.2013.00178. eCollection 2013. Front Immunol. 2013. PMID: 23847619 Free PMC article.
References
-
- Bishop W.R., Bell R.M. Attenuation of sn-1,2-diacylglycerol second messengers. J. Biol. Chem. 1986;261:12513–12519. - PubMed
-
- Bunting M., Tang W., Zimmerman G.A., McIntyre T.M., Prescott S.M. Molecular cloning and characterization of a novel human diacylglycerol kinase ζ. J. Biol. Chem. 1996;271:10230–10236. - PubMed
-
- Chang J.-S., Noh D.Y., Park I.A., Kim M.J., Song H., Ryu S.H., Suh P.-G. Overexpression of phospholipase C-γ1 in rat 3Y1 fibroblast cells leads to malignant transformation. Cancer Res. 1997;57:5465–5468. - PubMed
-
- Chin L., Tam A., Pomerantz M.W., Wong M., Holash J., Bardeesy N., Shen Q., O'Hagan R., Pantginis J., Zhou H. Essential role for oncogenic Ras in tumor maintenance. Nature. 1999;400:468–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

