Mechanism of topology simplification by type II DNA topoisomerases
- PMID: 11248029
- PMCID: PMC30604
- DOI: 10.1073/pnas.061029098
Mechanism of topology simplification by type II DNA topoisomerases
Abstract
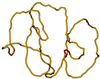
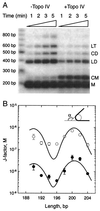
Type II DNA topoisomerases actively reduce the fractions of knotted and catenated circular DNA below thermodynamic equilibrium values. To explain this surprising finding, we designed a model in which topoisomerases introduce a sharp bend in DNA. Because the enzymes have a specific orientation relative to the bend, they act like Maxwell's demon, providing unidirectional strand passage. Quantitative analysis of the model by computer simulations proved that it can explain much of the experimental data. The required sharp DNA bend was demonstrated by a greatly increased cyclization of short DNA fragments from topoisomerase binding and by direct visualization with electron microscopy.
Figures
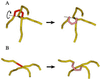
Similar articles
-
Simplification of DNA topology below equilibrium values by type II topoisomerases.Science. 1997 Aug 1;277(5326):690-3. doi: 10.1126/science.277.5326.690. Science. 1997. PMID: 9235892
-
Visualization of DNA topoisomerases by electron microscopy.Methods Mol Biol. 1999;94:269-77. doi: 10.1385/1-59259-259-7:269. Methods Mol Biol. 1999. PMID: 12844882 No abstract available.
-
Direct measurement of DNA bending by type IIA topoisomerases: implications for non-equilibrium topology simplification.Nucleic Acids Res. 2011 Jul;39(13):5729-43. doi: 10.1093/nar/gkr109. Epub 2011 Mar 17. Nucleic Acids Res. 2011. PMID: 21421557 Free PMC article.
-
[Distributions of circular DNA according its topological states].Mol Biol (Mosk). 2001 Mar-Apr;35(2):285-97. Mol Biol (Mosk). 2001. PMID: 11357411 Review. Russian.
-
Multiple bacterial topoisomerases: specialization or redundancy?Bioessays. 1993 Jul;15(7):445-9. doi: 10.1002/bies.950150703. Bioessays. 1993. PMID: 8397515 Review.
Cited by
-
What's on the Other Side of the Gate: A Structural Perspective on DNA Gate Opening of Type IA and IIA DNA Topoisomerases.Int J Mol Sci. 2023 Feb 16;24(4):3986. doi: 10.3390/ijms24043986. Int J Mol Sci. 2023. PMID: 36835394 Free PMC article. Review.
-
A target site for spontaneous insertion of IS10 element in pUC19 DNA located within intrinsically bent DNA.Open Microbiol J. 2009 Sep 25;3:146-50. doi: 10.2174/1874285800903010146. Open Microbiol J. 2009. PMID: 19812719 Free PMC article.
-
DNA topoisomerase II and its growing repertoire of biological functions.Nat Rev Cancer. 2009 May;9(5):327-37. doi: 10.1038/nrc2608. Epub 2009 Apr 20. Nat Rev Cancer. 2009. PMID: 19377505 Free PMC article. Review.
-
How do type II topoisomerases use ATP hydrolysis to simplify DNA topology beyond equilibrium? Investigating the relaxation reaction of nonsupercoiling type II topoisomerases.J Mol Biol. 2009 Feb 6;385(5):1397-408. doi: 10.1016/j.jmb.2008.11.056. Epub 2008 Dec 7. J Mol Biol. 2009. PMID: 19094994 Free PMC article.
-
DNA supercoiling inhibits DNA knotting.Nucleic Acids Res. 2008 Sep;36(15):4956-63. doi: 10.1093/nar/gkn467. Epub 2008 Jul 25. Nucleic Acids Res. 2008. PMID: 18658246 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

