Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper
- PMID: 11230127
- PMCID: PMC145500
- DOI: 10.1093/emboj/20.5.1033
Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper
Abstract
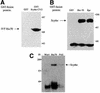
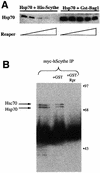
Protein folding mediated by the Hsp70 family of molecular chaperones requires both ATP and the co-chaperone Hdj-1. BAG-1 was recently identified as a bcl-2-interacting, anti-apoptotic protein that binds to the ATPase domain of Hsp70 and prevents the release of the substrate. While this suggested that cells had the potential to modulate Hsp70-mediated protein folding, physiological regulators of BAG-1 have yet to be identified. We report here that the apoptotic regulator Scythe, originally isolated through binding to the potent apoptotic inducer Reaper, shares limited sequence identity with BAG-1 and inhibits Hsp70- mediated protein refolding. Scythe-mediated inhibition of Hsp70 is reversed by Reaper, providing evidence for the regulated reversible inhibition of chaperone activity. As Scythe functions downstream of Reaper in apoptotic induction, these findings suggest that Scythe/Reaper may signal apoptosis, in part through regulating the folding and activity of apoptotic signaling molecules.
Figures









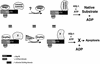
Similar articles
-
Scythe: a novel reaper-binding apoptotic regulator.EMBO J. 1998 Nov 2;17(21):6135-43. doi: 10.1093/emboj/17.21.6135. EMBO J. 1998. PMID: 9799223 Free PMC article.
-
Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity.EMBO J. 1999 Oct 15;18(20):5486-93. doi: 10.1093/emboj/18.20.5486. EMBO J. 1999. PMID: 10523293 Free PMC article.
-
Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper.Mol Biol Cell. 2003 Oct;14(10):4162-72. doi: 10.1091/mbc.e03-03-0139. Epub 2003 Jul 11. Mol Biol Cell. 2003. PMID: 14517326 Free PMC article.
-
Hsp70 chaperones: cellular functions and molecular mechanism.Cell Mol Life Sci. 2005 Mar;62(6):670-84. doi: 10.1007/s00018-004-4464-6. Cell Mol Life Sci. 2005. PMID: 15770419 Free PMC article. Review.
-
Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights.Biol Chem. 1998 Mar;379(3):269-74. Biol Chem. 1998. PMID: 9563821 Review.
Cited by
-
A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):16113-8. doi: 10.1073/pnas.2136610100. Epub 2003 Dec 11. Proc Natl Acad Sci U S A. 2003. PMID: 14671332 Free PMC article.
-
Scythe/BAT3 regulates apoptotic cell death induced by papillomavirus binding factor in human osteosarcoma.Cancer Sci. 2009 Jan;100(1):47-53. doi: 10.1111/j.1349-7006.2008.00991.x. Epub 2008 Oct 29. Cancer Sci. 2009. PMID: 19018758 Free PMC article.
-
Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis.J Cell Biol. 2008 Aug 11;182(3):449-58. doi: 10.1083/jcb.200802113. Epub 2008 Aug 4. J Cell Biol. 2008. PMID: 18678708 Free PMC article.
-
CLEC14a-HSP70-1A interaction regulates HSP70-1A-induced angiogenesis.Sci Rep. 2017 Sep 6;7(1):10666. doi: 10.1038/s41598-017-11118-y. Sci Rep. 2017. PMID: 28878328 Free PMC article.
-
Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion.Nat Med. 2012 Sep;18(9):1394-400. doi: 10.1038/nm.2871. Nat Med. 2012. PMID: 22863785 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

