A di-leucine sequence and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts
- PMID: 11179421
- PMCID: PMC30949
- DOI: 10.1091/mbc.12.2.367
A di-leucine sequence and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts
Abstract
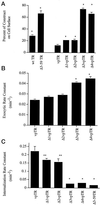
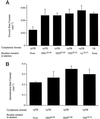
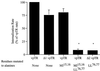
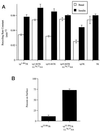
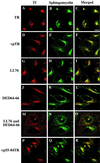
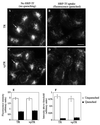
Insulin-regulated aminopeptidase (IRAP), a transmembrane aminopeptidase, is dynamically retained within the endosomal compartment of fibroblasts. The characteristics of this dynamic retention are rapid internalization from the plasma membrane and slow recycling back to the cell surface. These specialized trafficking kinetics result in <15% of IRAP on the cell surface at steady state, compared with 35% of the transferrin receptor, another transmembrane protein that traffics between endosomes and the cell surface. Here we demonstrate that a 29-amino acid region of IRAP's cytoplasmic domain (residues 56--84) is necessary and sufficient to promote trafficking characteristic of IRAP. A di-leucine sequence and a cluster of acidic amino acids within this region are essential elements of the motif that slows IRAP recycling. Rapid internalization requires any two of three distinct motifs: M(15,16), DED(64--66), and LL(76,77). The DED and LL sequences are part of the motif that regulates recycling, demonstrating that this motif is bifunctional. In this study we used horseradish peroxidase quenching of fluorescence to demonstrate that IRAP is dynamically retained within the transferrin receptor-containing general endosomal recycling compartment. Therefore, our data demonstrate that motifs similar to those that determine targeting among distinct membrane compartments can also regulate the rate of transport of proteins from endosomal compartments. We propose a model for dynamic retention in which IRAP is transported from the general endosomal recycling compartment in specialized, slowly budding recycling vesicles that are distinct from those that mediate rapid recycling back to the surface (e.g., transferrin receptor-containing transport vesicles). It is likely that the dynamic retention of IRAP is an example of a general mechanism for regulating the distribution of proteins between the surface and interior of cells.
Figures

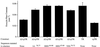
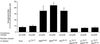

Similar articles
-
Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles.Mol Biol Cell. 2001 Nov;12(11):3489-501. doi: 10.1091/mbc.12.11.3489. Mol Biol Cell. 2001. PMID: 11694583 Free PMC article.
-
Identification of an insulin-responsive, slow endocytic recycling mechanism in Chinese hamster ovary cells.J Biol Chem. 1998 Jul 10;273(28):17968-77. doi: 10.1074/jbc.273.28.17968. J Biol Chem. 1998. PMID: 9651404
-
Insulin-regulated aminopeptidase marks an antigen-stimulated recycling compartment in mast cells.Traffic. 2006 Feb;7(2):155-67. doi: 10.1111/j.1600-0854.2006.00373.x. Traffic. 2006. PMID: 16420524
-
Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes.Biol Pharm Bull. 2004 Jun;27(6):761-4. doi: 10.1248/bpb.27.761. Biol Pharm Bull. 2004. PMID: 15187412 Review.
-
The Role of Insulin Regulated Aminopeptidase in Endocytic Trafficking and Receptor Signaling in Immune Cells.Front Mol Biosci. 2020 Oct 20;7:583556. doi: 10.3389/fmolb.2020.583556. eCollection 2020. Front Mol Biosci. 2020. PMID: 33195428 Free PMC article. Review.
Cited by
-
Alternative splicing results in RET isoforms with distinct trafficking properties.Mol Biol Cell. 2012 Oct;23(19):3838-50. doi: 10.1091/mbc.E12-02-0114. Epub 2012 Aug 8. Mol Biol Cell. 2012. PMID: 22875993 Free PMC article.
-
A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane.EMBO J. 2002 Jun 3;21(11):2557-67. doi: 10.1093/emboj/21.11.2557. EMBO J. 2002. PMID: 12032069 Free PMC article.
-
Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function.Biochem J. 2002 Jun 15;364(Pt 3):777-86. doi: 10.1042/BJ20011361. Biochem J. 2002. PMID: 12049642 Free PMC article.
-
Improvement in lipid and protein trafficking in Niemann-Pick C1 cells by correction of a secondary enzyme defect.Traffic. 2010 May;11(5):601-15. doi: 10.1111/j.1600-0854.2010.01046.x. Epub 2010 Feb 22. Traffic. 2010. PMID: 20412078 Free PMC article.
-
MAST4 promotes primary ciliary resorption through phosphorylation of Tctex-1.Life Sci Alliance. 2023 Sep 19;6(11):e202301947. doi: 10.26508/lsa.202301947. Print 2023 Nov. Life Sci Alliance. 2023. PMID: 37726137 Free PMC article.
References
-
- Andersson H, Kappeler F, Hauri H-P. Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J Biol Chem. 1999;274:15080–15084. - PubMed
-
- Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. - PubMed
-
- Diaz E, Pfeffer S. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

