Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation
- PMID: 11172047
- PMCID: PMC29353
- DOI: 10.1073/pnas.98.4.1895
Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation
Abstract
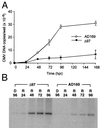
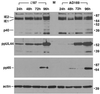
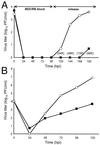
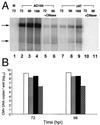
The human cytomegalovirus UL97 kinase, an important target of antiviral therapy, has an impact on at least two distinct phases of viral replication. Compared with wild-type virus, the UL97 deletion mutant exhibits an early replication defect that reduces DNA accumulation by 4- to 6-fold, as well as a late capsid maturation defect responsible for most of the observed 100- to 1000-fold reduction in replication. Block-release experiments with the antiviral 2-bromo-5,6-dichloro-1-(beta-D-ribofuranosyl)-benzimidazole revealed an important role for UL97 kinase in capsid assembly. Although cleavage of concatemeric DNA intermediates to unit-length genomes remained unaffected, progeny mutant virus maturation was delayed, with accumulation of progeny at significantly reduced levels compared with wild type after release of this block. Transmission electron microscopy confirmed the aberrant accumulation of empty A-like capsids containing neither viral DNA nor an internal scaffold structure, consistent with a failure to stably package DNA in mutant virus-infected cells. The function of UL97 in DNA synthesis as well as capsid assembly suggests that protein phosphorylation mediated by this herpesvirus-conserved kinase increases the efficiency of these two distinct phases of virus replication.
Figures

Similar articles
-
Human Cytomegalovirus Can Procure Deoxyribonucleotides for Viral DNA Replication in the Absence of Retinoblastoma Protein Phosphorylation.J Virol. 2016 Sep 12;90(19):8634-43. doi: 10.1128/JVI.00731-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440891 Free PMC article.
-
An epistatic relationship between the viral protein kinase UL97 and the UL133-UL138 latency locus during the human cytomegalovirus lytic cycle.J Virol. 2014 Jun;88(11):6047-60. doi: 10.1128/JVI.00447-14. Epub 2014 Mar 12. J Virol. 2014. PMID: 24623439 Free PMC article.
-
Human cytomegalovirus UL97 kinase and nonkinase functions mediate viral cytoplasmic secondary envelopment.J Virol. 2011 Apr;85(7):3375-84. doi: 10.1128/JVI.01952-10. Epub 2011 Jan 19. J Virol. 2011. PMID: 21248036 Free PMC article.
-
Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir.Rev Med Virol. 2009 Jul;19(4):215-29. doi: 10.1002/rmv.615. Rev Med Virol. 2009. PMID: 19434630 Free PMC article. Review.
-
[The mechanisms of human cytomegalovirus DNA replication].Nihon Rinsho. 1998 Jan;56(1):36-43. Nihon Rinsho. 1998. PMID: 9465662 Review. Japanese.
Cited by
-
The absence of p53 during Human Cytomegalovirus infection leads to decreased UL53 expression, disrupting UL50 localization to the inner nuclear membrane, and thereby inhibiting capsid nuclear egress.Virology. 2016 Oct;497:262-278. doi: 10.1016/j.virol.2016.07.020. Epub 2016 Aug 4. Virology. 2016. PMID: 27498409 Free PMC article.
-
Human cytomegalovirus resistance to antiviral drugs.Antimicrob Agents Chemother. 2005 Mar;49(3):873-83. doi: 10.1128/AAC.49.3.873-883.2005. Antimicrob Agents Chemother. 2005. PMID: 15728878 Free PMC article. Review. No abstract available.
-
Inhibition of human cytomegalovirus replication by benzimidazole nucleosides involves three distinct mechanisms.Antimicrob Agents Chemother. 2004 Oct;48(10):3918-27. doi: 10.1128/AAC.48.10.3918-3927.2004. Antimicrob Agents Chemother. 2004. PMID: 15388453 Free PMC article.
-
Human cytomegalovirus UL97 Kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis.J Virol. 2005 Dec;79(24):15494-502. doi: 10.1128/JVI.79.24.15494-15502.2005. J Virol. 2005. PMID: 16306620 Free PMC article.
-
Functional map of human cytomegalovirus AD169 defined by global mutational analysis.Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12396-401. doi: 10.1073/pnas.1635160100. Epub 2003 Sep 30. Proc Natl Acad Sci U S A. 2003. PMID: 14519856 Free PMC article.
References
-
- Alford C A, Britt W J. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Lippincott-Raven; 1996. pp. 2493–2534.
-
- Mocarski E S, Jr, Tan Courcelle C. In: Fields Virology. Knipe D M, Howley P M, editors. Philadelphia: Lippincott Williams & Wilkens; 2001. , in press.
-
- Black L W. Annu Rev Microbiol. 1989;43:267–292. - PubMed
-
- Steven A C, Spear P G. In: Structural Biology of Viruses. Chiu W, Burnett R M, Garcea R L, editors. Oxford: Oxford Univ. Press; 1997. pp. 312–351.
-
- Gibson W. Intervirology. 1996;39:389–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

