A human myeloma cell line suitable for the generation of human monoclonal antibodies
- PMID: 11172031
- PMCID: PMC29337
- DOI: 10.1073/pnas.98.4.1799
A human myeloma cell line suitable for the generation of human monoclonal antibodies
Abstract
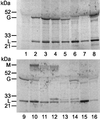
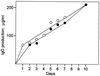
Ever since monoclonal antibodies were produced in 1975 with mouse myeloma cells there has been interest in developing human myeloma cultures for the production of monoclonal antibodies. However, despite multiple attempts, no human myeloma line suitable for hybridoma production has been described. Here we report the derivation of a hypoxanthine-aminopterin-thymidine-sensitive and ouabain-resistant human myeloma cell line (Karpas 707H) that contains unique genetic markers. We show that this line is useful for the generation of stable human hybridomas. It can easily be fused with ouabain-sensitive Epstein-Barr virus-transformed cells as well as with fresh tonsil and blood lymphocytes, giving rise to stable hybrids that continuously secrete very large quantities of human immunoglobulins. The derived hybrids do not lose immunoglobulin secretion over many months of continuous growth. The availability of this cell line should enable the in vitro immortalization of human antibody-producing B cells that are formed in vivo. The monoclonal antibodies produced may have advantages in immunotherapy.
Figures



Similar articles
-
Production of anti-tumor human monoclonal antibodies using different approaches.Hum Antibodies Hybridomas. 1993 Jan;4(1):2-8. Hum Antibodies Hybridomas. 1993. PMID: 8381684
-
SPAM-8, a mouse-human heteromyeloma fusion partner in the production of human monoclonal antibodies. Establishment of a human monoclonal antibody against cytomegalovirus.Hum Antibodies Hybridomas. 1991 Jan;2(1):26-32. Hum Antibodies Hybridomas. 1991. PMID: 1651784
-
Characterization of stable Epstein-Barr (EB) virus transformed cell lines and mouse-human hybridomas producing a large quantity of anti-tetanus toxoid (TT) monoclonal antibody.Behring Inst Mitt. 1985 Dec;(78):139-47. Behring Inst Mitt. 1985. PMID: 3008706
-
Monoclonal antibodies: methods and clinical laboratory applications.Clin Physiol Biochem. 1983;1(2-5):160-72. Clin Physiol Biochem. 1983. PMID: 6396016 Review.
-
Human T-T cell hybridomas: development and applications.Hum Antibodies Hybridomas. 1990;1(1):3-9. Hum Antibodies Hybridomas. 1990. PMID: 2103350 Review.
Cited by
-
Demystified...recombinant antibodies.J Clin Pathol. 2004 Sep;57(9):912-7. doi: 10.1136/jcp.2003.014407. J Clin Pathol. 2004. PMID: 15333649 Free PMC article. Review.
-
Exploring the native human antibody repertoire to create antiviral therapeutics.Curr Top Microbiol Immunol. 2008;317:155-83. doi: 10.1007/978-3-540-72146-8_6. Curr Top Microbiol Immunol. 2008. PMID: 17990793 Free PMC article. Review.
-
Mining human antibody repertoires.MAbs. 2010 Jul-Aug;2(4):365-78. doi: 10.4161/mabs.12187. Epub 2010 Jul 1. MAbs. 2010. PMID: 20505349 Free PMC article. Review.
-
Is cancer progression caused by gradual or simultaneous acquisitions of new chromosomes?Mol Cytogenet. 2018 Jan 15;11:4. doi: 10.1186/s13039-017-0350-4. eCollection 2018. Mol Cytogenet. 2018. PMID: 29371887 Free PMC article.
-
Narrative review of the novel coronavirus SARS-CoV-2: update on genomic characteristics, transmissions and animal model.J Thorac Dis. 2020 Dec;12(12):7454-7466. doi: 10.21037/jtd-20-2084. J Thorac Dis. 2020. PMID: 33447433 Free PMC article. Review.
References
-
- Cotton R G H, Milstein C. Nature (London) 1973;244:42–43. - PubMed
-
- Köhler G, Milstein C. Nature (London) 1975;256:495–497. - PubMed
-
- Gustafsson B, Hinkula J. Hum Antibod Hybridomas. 1994;5:98–104. - PubMed
-
- Lloyd-Evans P, Gilmour J E M. Hybridoma. 2000;19:143–149. - PubMed
-
- Nilsson K. Int J Cancer. 1971;7:380–396. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

